Fluid and Electrolyte Balance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Calciphylaxis with Normal Renal and Parathyroid Function Not As Rare As Previously Believed
OBSERVATION Calciphylaxis With Normal Renal and Parathyroid Function Not as Rare as Previously Believed Andrew H. Kalajian, MD; Paula S. Malhotra, MD; Jeffrey P. Callen, MD; Lynn P. Parker, MD Background: Calciphylaxis is a life-threatening form of previously reported cases of nontraditional calciphylaxis metastatic calcification-induced microvascular occlusion identified the following patient characteristics that high- syndrome. Although traditionally observed in patients with light clinical situations potentially predisposing to calci- end-stage renal disease and/or hyperparathyroidism, the phylaxis: hypoalbuminemia, malignant neoplasm, sys- development of calciphylaxis in “nontraditional” pa- temic corticosteroid use, anticoagulation with warfarin tients having both normal renal and parathyroid func- sodium or phenprocoumon, chemotherapy, systemic in- tion has been reported. However, to date there has been flammation, hepatic cirrhosis, protein C or S deficiency, no collective analysis identifying common patient char- obesity, rapid weight loss, and infection. acteristics potentially predisposing to the development of calciphylaxis in nontraditional patients. Conclusions: Calciphylaxis is becoming increasingly common in patients with normal renal and parathyroid Observations: A 58-year-old woman with endometrial function. The observations from this study may assist der- carcinoma developed extensive calciphylaxis despite the matologists in the rapid diagnosis and prompt initiation presence of normal renal and parathyroid function. -

Soft Tissue Calcification and Ossification
Soft Tissue Calcification and Ossification Soft-tissue Calcification Metastatic Calcification =deposit of calcium salts in previously normal tissue (1) as a result of elevation of Ca x P product above 60-70 (2) with normal Ca x P product after renal transplant Location:lung (alveolar septa, bronchial wall, vessel wall), kidney, gastric mucosa, heart, peripheral vessels Cause: (a)Skeletal deossification 1.1° HPT 2.Ectopic HPT production (lung / kidney tumor) 3.Renal osteodystrophy + 2° HPT 4.Hypoparathyroidism (b)Massive bone destruction 1.Widespread bone metastases 2.Plasma cell myeloma 3.Leukemia Dystrophic Calcification (c)Increased intestinal absorption =in presence of normal serum Ca + P levels secondary to local electrolyte / enzyme alterations in areas of tissue injury 1.Hypervitaminosis D Cause: 2.Milk-alkali syndrome (a)Metabolic disorder without hypercalcemia 3.Excess ingestion / IV administration of calcium salts 1.Renal osteodystrophy with 2° HPT 4.Prolonged immobilization 2.Hypoparathyroidism 5.Sarcoidosis 3.Pseudohypoparathyroidism (d)Idiopathic hypercalcemia 4.Pseudopseudohypoparathyroidism 5.Gout 6.Pseudogout = chondrocalcinosis 7.Ochronosis = alkaptonuria 8.Diabetes mellitus (b) Connective tissue disorder 1.Scleroderma 2.Dermatomyositis 3.Systemic lupus erythematosus (c)Trauma 1.Neuropathic calcifications 2.Frostbite 3.Myositis ossificans progressiva 4.Calcific tendinitis / bursitis (d)Infestation 1.Cysticercosis Generalized Calcinosis 2.Dracunculosis (guinea worm) (a)Collagen vascular disorders 3.Loiasis 1.Scleroderma -

Electrolyte Disorders in Cancer Patients: a Systematic Review
Berardi et al. J Cancer Metastasis Treat 2019;5:79 Journal of Cancer DOI: 10.20517/2394-4722.2019.008 Metastasis and Treatment Review Open Access Electrolyte disorders in cancer patients: a systematic review Rossana Berardi, Mariangela Torniai, Edoardo Lenci, Federica Pecci, Francesca Morgese, Silvia Rinaldi Clinica Oncologica, Università Politecnica delle Marche, Azienda Ospedaliero-Universitaria Ospedali Riuniti Umberto I - GM Lancisi - G Salesi, Ancona 60126, Italy. Correspondence to: Prof. Rossana Berardi, Clinica Oncologica, Università Politecnica delle Marche, Azienda Ospedaliero- Universitaria Ospedali Riuniti di Ancona, Via Conca 71, Ancona 60126, Italy. E-mail: [email protected] How to cite this article: Berardi R, Torniai M, Lenci E, Pecci F, Morgese F, Rinaldi S. Electrolyte disorders in cancer patients: a systematic review. J Cancer Metastasis Treat 2019;5:79. http://dx.doi.org/10.20517/2394-4722.2019.008 Received: 26 Apr 2019 First Decision: 26 Jul 2019 Revised: 20 Nov 2019 Accepted: 20 Nov 2019 Published: 9 Dec 2019 Science Editor: Stephen J. Ralph Copy Editor: Jing-Wen Zhang Production Editor: Jing Yu Abstract Electrolyte disorders are very common complications in cancer patients. They might be associated to a worsening outcome, influencing quality of life, possibility to receive anticancer drugs, and conditioning survival. In fact, they might provoke important morbidity, with dysfunction of multiple organs and rarely causing life-threatening conditions. Moreover, recent studies showed that they might worsen cancer patients’ outcome, while a prompt correction seems to have a positive impact. Furthermore, there is evidence of a correlation between electrolyte alterations and poorer performance status, delays in therapy commencement and continuation, and negative treatment outcomes. -

T PATHOLOGIC CALCIFICATION Deposition of Calcium Salts In
t PATHOLOGIC CALCIFICATION Deposition of calcium salts in tissues other than osteoid or enamel is called pathologic or heterotopic calcification. Two distinct types of pathologic calcification are recognised: Dystrophic calcification, which is characterised by deposition of calcium salts in dead or degenerated tissues with normal calcium metabolism and normal serum calcium levels. Dystrophic calcification may occur due to 2 types of causes: ● Calcification in dead tissue ● Calcification of degenerated tissue Metastatic calcification, on the other hand, occurs in apparently normal tissues and is associated with deranged calcium metabolism and hypercalcaemia. Since metastatic calcification occurs in normal tissues due to hypercalcaemia, its causes would include one of the following two conditions: ● Excessive mobilisation of calcium from the bone. ● Excessive absorption of calcium from the gut. Pathogenesis of Dystrophic Calcification The process of dystrophic calcification has been likened to the formation of normal hydroxyapatite in the bone involving 2 phases: ● Initiation and propagation: Initiation is the phase in which precipitates of calcium phosphate begin to accumulate intracellularly in the mitochondria, or extracellularly in membrane-bound vesicles. Propagation is the phase in which minerals deposited in the initiation phase are propagated to form mineral crystals. Pathogenesis of metastatic calcification Metastatic calcification occurs due to excessive binding of inorganic phosphate ions with calcium ions, which are elevated due to underlying metabolic derangement. This leads to formation of precipitates of calcium phosphate at the preferential sites. Metastatic calcification is reversible upon correction of underlying metabolic disorder. GANGRENE Gangrene is a form of necrosis of tissue with superadded putrefaction. The type of necrosis is usually coagulative due to ischaemia (e.g. -

Parenteral Nutrition Primer: Balance Acid-Base, Fluid and Electrolytes
Parenteral Nutrition Primer: Balancing Acid-Base, Fluids and Electrolytes Phil Ayers, PharmD, BCNSP, FASHP Todd W. Canada, PharmD, BCNSP, FASHP, FTSHP Michael Kraft, PharmD, BCNSP Gordon S. Sacks, Pharm.D., BCNSP, FCCP Disclosure . The program chair and presenters for this continuing education activity have reported no relevant financial relationships, except: . Phil Ayers - ASPEN: Board Member/Advisory Panel; B Braun: Consultant; Baxter: Consultant; Fresenius Kabi: Consultant; Janssen: Consultant; Mallinckrodt: Consultant . Todd Canada - Fresenius Kabi: Board Member/Advisory Panel, Consultant, Speaker's Bureau • Michael Kraft - Rockwell Medical: Consultant; Fresenius Kabi: Advisory Board; B. Braun: Advisory Board; Takeda Pharmaceuticals: Speaker’s Bureau (spouse) . Gordon Sacks - Grant Support: Fresenius Kabi Sodium Disorders and Fluid Balance Gordon S. Sacks, Pharm.D., BCNSP Professor and Department Head Department of Pharmacy Practice Harrison School of Pharmacy Auburn University Learning Objectives Upon completion of this session, the learner will be able to: 1. Differentiate between hypovolemic, euvolemic, and hypervolemic hyponatremia 2. Recommend appropriate changes in nutrition support formulations when hyponatremia occurs 3. Identify drug-induced causes of hypo- and hypernatremia No sodium for you! Presentation Outline . Overview of sodium and water . Dehydration vs. Volume Depletion . Water requirements & Equations . Hyponatremia • Hypotonic o Hypovolemic o Euvolemic o Hypervolemic . Hypernatremia • Hypovolemic • Euvolemic • Hypervolemic Sodium and Fluid Balance . Helpful hint: total body sodium determines volume status, not sodium status . Examples of this concept • Hypervolemic – too much volume • Hypovolemic – too little volume • Euvolemic – normal volume Water Distribution . Total body water content varies from 50-70% of body weight • Dependent on lean body mass: fat ratio o Fat water content is ~10% compared to ~75% for muscle mass . -
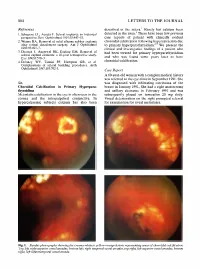
Sir, Metastatic Calcification in the Eye Is Often Seen in the Cornea and the Interpalpebral Conjunctiva. in Hypercalcaemic Subje
804 LETTERS TO THE JOURNAL References described in the sclera.1 Rarely has calcium been 1 1. Schepens CL, Acosta F. Scleral implants: an historical detected in the uvea. There have been few previous perspective. Surv OphthalmoI1991;35:447-53. case reports of patient with clinically evident 2. Wizina RA. Removal of solid silicone rubber explants choroidal calcification following hypercalcaemia due after retinal detachment surgery. Am J Ophthalmol to primary hyperparathyroidism 2. We present the 1983;95:495-7. clinical and investigative findings of a patient who 3. Deutsch J, Aggarwal RK, Eagling EM. Removal of had been treated for primary hyperparathyroidism · scleral explant elements: a 10-year retrospective study. and who was found some years later to have Eye 1992;6:570-3. choroidal calcification. 4. Delaney WV, Tomisi PF, Hampton GR, et al. Complications of scleral buckling procedures. Arch OphthalmoI1987;105:702-3. Case Report A 60-year-old woman with a complex medical history was referred to the eye clinic in September 1991. She Sir, was diagnosed with infiltrating carcinoma of the Choroidal Calcification in Primary Hyperpara breast in January 1991. She had a right mastectomy thyroidism and axillary clearance in February 1991 and was Metastatic calcificationin the eye is often seen in the subsequently placed on tamoxifen 20 mg daily. cornea and the interpalpebral conjunctiva. In Visual deterioration on the right prompted referral hypercalcaemic subjects calcium has also been for examination for uveal metastases. Fig. 1. Fundus photographs showing the creamy-white to yellow-orange lesions representing areas of choroidal calcification: Top left, right superior vessel arcades; bottom left, right temporal vessel arcades; top right, left superior vessel arcades; bottom right, left inferotemporal vessel arcade. -

Understanding Calcinosis and Calciphylaxis
PRACTICE DEVELOPMENT Understanding calcinosis and calciphylaxis KEY WORDS Calcinosis cutis is a rare cause of non-healing leg ulceration. There are many factors Calcinosis cutis that can delay the healing of venous leg ulceration and the deposition of calcium in Calciphylaxis the skin known as calcinosis cutis is one of these factors. There are five distinct forms: Warfarin-induced skin dystrophic calcification, metastatic calcification, idiopathic calcification, iatrogenic necrosis calcification and calciphylaxis. Warfarin skin necrosis has common clinical features with calciphylaxis and is therefore included in this article, which describes the types of calcinosis cutis, their clinical presentations and limited treatment options. The aim is to highlight these unusual causes and to assist healthcare professionals when faced with a non-healing ulcer. eg ulceration can be defined as a defect in neurotransmission and the blood coagulation the dermis located on the leg (Franks et al, pathway. At a cellular level, it is implicated in 2016). Leg ulceration is a significant clinical cell-to-cell communication (Walshe and Fairley, Lproblem with the majority attributing venous 1995). In the skin, it is specifically concerned with hypertension as the underlying disease process with keratinocyte proliferation, differentiation and venous leg ulceration affecting 1% of the population adhesion (Smith and Yamada, 2002). in the western world (Posnett et al, 2009). However, The level of serum calcium is closely there is a multitude of causative factors of leg ulcers, controlled by the parathyroid hormone. with the term leg ulcer purely signifying the clinical Regardless of this regulation, it is possible for manifestation and not the underlying aetiology. -

Adverse Renal and Metabolic Effects Associated with Oral Sodium Phosphate Bowel Preparation
CJASN ePress. Published on July 2, 2008 as doi: 10.2215/CJN.02040408 In-Depth Review Adverse Renal and Metabolic Effects Associated with Oral Sodium Phosphate Bowel Preparation Eliot C. Heher,* Samuel O. Thier,*† Helmut Rennke,‡ and Benjamin D. Humphreys§ *Department of Medicine, Division of Nephrology, Massachusetts General Hospital, Boston, Massachusetts; †Department of Medicine and Healthcare Policy, Harvard Medical School, Boston, Massachusetts; ‡Renal Pathology and §Renal Division, Brigham and Women’s Hospital, Boston, Massachusetts Colorectal cancer can be prevented by the removal of adenomatous polyps during screening colonoscopy, but adequate bowel preparation is required. Oral sodium phosphate (OSP), an effective bowel purgative, is available over the counter and requires a substantially lower volume than polyethylene glycol-based preparative agents. Accumulating reports implicate OSP in electrolyte disturbances as well as acute kidney injury (AKI) in a syndrome termed phosphate nephropathy (a form of nephrocalcinosis). Despite published case reports and case series, the actual incidence, risk factors, and natural history of phosphate nephropathy remain largely undefined. Several recent observational studies have provided new information on these important issues while supporting a link between OSP and acute phosphate nephropathy as well as the development of chronic kidney disease in elderly patients, many of whom had a normal serum creatinine at the time of OSP ingestion. This review summarizes current knowledge about the renal complications of OSP, risk factors for its development, and the pathophysiology of acute and chronic kidney damage in nephrocalcinosis. Clin J Am Soc Nephrol ●●: ●●●-●●●, 2008. doi: 10.2215/CJN.02040408 pproximately 14 million colonoscopies are performed known as Nu-Lytely, formulated without the unpalatable in- in the United States yearly for colon cancer screening, gredient sodium sulfate, became available in 1990 (9). -

Hypercalcemia
Hypercalcemia Definition: 1. Hypercalcemiaypercalcemia is defined as a serum calcium > 10.5 mg/dlmg/dl and severe hypercalemia is defined as a serum calcium > 14 mg/dlmg/dl (> 3.5 mmol/L)mmol/L).... 2. A hypercalcemic crisis is present when severe neurological symptoms or cardiac arrhythmias are present in a patient with a serum calcium > 14 mg/dl (> 3.5 mmol/L) or when the serum calcium is > 16 mg/dl (> 4 mmol/L) I Causes A. Malignancy 1. Breast Cancer with bone metastases 2. Lung Cancer 3. Head and Neck squamous cell cancer 4. Renal Cell Cancer 5. Hematologic a. Multiple Myeloma b. Hodgkin's Lymphoma B. Paget's Disease of Bone C. Hyperparathyroidism 1. Primary Hyperparathyroidism (most common cause) a. May be associated with multiple endocrine neoplasia 2. Tertiary Hyperparathyroidism D. Medications 1. Thiazide Diuretic s 2. Lithium 3. Vitamin A toxicity 4. Vitamin D toxicity (e.g. 25-Hydroxyvitamin D2) 5. Milk alkali syndrome E. Endocrine 1. Adrenal Insufficiency 2. Thyrotoxicosis ( Hyperthyroidism ) 3. Pheochromocytoma 4. Acromegaly F. Other causes 1. Familial hypocalciuric hypercalcemia 2. Prolonged immobilization 3. Granulomatous disease ( Sarcoidosis , Tuberculosis ) II. Symptoms and Signs A. Often asymptomatic B. Symptoms and Signs are related to Serum Calcium Levels 1. Calcium > 11.5 mg/dl (2.9 mmol/L) a. Symptom onset 2. Calcium > 13 mg/dl (3.2 mmol/L) a. Nephrocalcinosis b. Acute Renal Failure C. General Symptoms 1. See complication-specific symptoms below 2. Nausea 3. Headache 4. Diarrhea 5. Anorexia 6. Lethargy 7. Pruritus (Metastatic calcification of skin) III. Imaging A. Calcified soft tissues IV. -

Calciphylaxis
Postgrad Med J 2001;77:557–561 557 Postgrad Med J: first published as 10.1136/pmj.77.911.557 on 1 September 2001. Downloaded from REVIEWS Calciphylaxis R V Mathur, J R Shortland, A M El Nahas Abstract phosphate diet act as sensitising agents result- The phenomenon of calciphylaxis is rare, ing in a high calcium-phosphate product (Ca × but potentially fatal. It has been recog- P; normal range 4.2–5.6 mmol2/l2) with result- nised for a long time in patients with ant precipitation of Ca-P crystals.14 This results chronic renal failure with secondary hy- in diVuse calcification of the media and perparathyroidism. Disturbed calcium internal elastic lamina of small to medium and phosphate metabolism can result in sized arteries and arterioles with intimal prolif- painful necrosis of skin, subcutaneous tis- eration and rarely arterial occlusion causing sue and acral gangrene. Appearance of the tissue necrosis. This entity was given the term lesions is distinctive but the pathogenesis calcinosis cutis to signify vascular calcification remains uncertain. The beneficial eVects with ischaemic epidermolysis as seen in of parathyroidectomy are controversial. patients with chronic renal failure on However, correction of hyperphosphatae- haemodialysis.15–17 Other triggering events in- mia or occasionally hypercalcaemia is clude intravenous iron dextran and albumin imperative. Fulminant sepsis as a conse- infusion, low serum albumin, corticosteroids, quence of secondary infection of necrotic immunosuppression, trauma, subcutaneous in- and gangrenous tissue is a frequent cause jections in obese patients, and protein C and S of patient morbidity and mortality. deficiencies causing hypercoagulability and 8 12 16 18–25 (Postgrad Med J 2001;77:557–561) subsequent thrombosis. -

Rapid Progression of Cardiac Calcification in a Patient with Hemodialysis
pISSN 1975-4612/ eISSN 2005-9655 Copyright © 2012 Korean Society of Echocardiography http://dx.doi.org/10.4250/jcu.2012.20.4.193 www.kse-jcu.org CASE REPORT J Cardiovasc Ultrasound 2012;20(4):193-196 Porcelain Heart: Rapid Progression of Cardiac Calcification in a Patient with Hemodialysis Hyeon-Uk Lee, MD1, Ho-Joong Youn, MD2, Byung-Ju Shim, MD1, Seung-Jae Lee, MD1, Mi-Youn Park, MD1, Jin-Uk Jeong, MD1, Gwan-Min Gu, MD3, Hui-Kyung Jeon, MD4, Ji-eun Lee, MD1 and Byung-Jin Kwon, MD1 1Division of Cardiology, Department of Internal Medicine, Pohang St. Mary’s Hospital, Pohang, Korea 2Division of Cardiology, Department of Internal Medicine, Seoul St. Mary’s Hospital, School of Medicine, The Catholic University of Korea, Seoul, Korea 3Department of Radiology, Pohang St. Mary’s Hospital, Pohang, Korea 4Division of Cardiology, Department of Internal Medicine, Uijeongbu St. Mary’s Hospital, School of Medicine, The Catholic University of Korea, Uijeongbu, Korea Cardiac calcification usually occurs in patients with end-stage renal disease. However, rapid progression of cardiac calcification is rarely associated with secondary hyperparathyroidism of end-stage renal disease. We report a patient with end-stage renal disease who showed moderate left ventricular hypertrophy at the first echocardiography, and showed severe myocardial calcification and severe mitral valve stenosis 4 years later. We suspected a rapid progression ‘porcelain heart’ cardiomyopathy secondary to hyperparathyroidism of end-stage renal disease. The patient underwent parathyroidectomy, and considered mitral valve replacement. KEY WORDS: End stage renal disease · Secondary hyperparathyroidism · Myocardial calcification · Mitral valve stenosis. Introduction ease. Here, we report our case with a review of the literature. -

Calcinosis Universalis
IMAGES Dx Calcinosis Universalis 1 Nasim Afsarmanesh, MD 2 Alan Gorn, MD 1 Department of Internal Medicine, University of California Los Angeles, Los Angeles, California 2 Department of Rheumatology, University of California Los Angeles, Los Angeles, California 38-year-old woman with juvenile dermato- debilitating secondary complications such as skin A myositis (JDM) and calcinosis universalis pre- ulcerations expressing calcified material, superim- sented with 3 days of drainage from a lesion on posed infections of skin lesions, joint contractures her right elbow. An examination of the elbow with severe arthralgias, and muscle atrophy. Calci- revealed diffuse and firm subcutaneous nodules nosis has been correlated with severity of JDM with overlying erythema. X-rays illustrated soft-tis- with presence of cardiac involvement and use of sue calcifications in the forearm and elbow with- more than one immunosuppression medication.1 out evidence of osteomyelitis (Figure 1). Wound It has also been associated with the degree of vas- cultures grew Staphylococcus aureus, and the culopathy and delay in initiation of therapy for patient was started on intravenous antibiotics for controlling inflammation in JDM.2 abscess treatment. Soft-tissue calcification can be classified into 5 Calcinosis universalis is soft-tissue calcifica- categories: tion presenting as a complication of JDM. It is of- 1. Dystrophic calcification occurs in injured tissues ten detected in childhood in 30% to 70% of with normal calcium, phosphorus, and parathyroid patients. It is hypothesized that calcinosis is due hormone levels, as seen in this patient. Calcified to chronic tissue inflammation, as seen in JDM, nodules or plaques occur in the extremities and but- leading to muscle damage, releasing calcium, and tocks.