Initial Evaluation for Type 1 Diabetes (T1DM)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bronson Healthcare Midwest Epic Review of Systems 10.3
Bronson HealthCare Midwest Epic Review of Systems 10.3 Constitution Endocrine Activity Change Y N Cold intolerance Y N Appetite Change Y N Heat intolerance Y N Chills Y N Polydipsia Y N Diaphoresis Y N Polyuria Y N Fatigue Y N GU Fever Y N Difficulty urinating Y N Unexpctd wt chnge Y N Dyspareunia Y N HENT Dysuria Y N Facial Swelling Y N Enuresis Y N Neck pain Y N Flank pain Y N Neck stiffness Y N Frequency Y N Ear Discharge Y N Genital Sore Y N Hearing loss Y N Hematuria Y N Ear pain Y N Menstrual problem Y N Tinnitus Y N Pelvic pain Y N Nosebleeds Y N Urgency Y N Congestion Y N Urine decreased Y N Rhinorrhea Y N Vaginal bleeding Y N Postnasal drip Y N Vaginal discharge Y N Sneezing Y N Vaginal pain Y N Sinus Pressure Y N Musc Dental problem Y N Arthralgias Y N Drooling Y N Back pain Y N Mouth sores Y N Gait problem Y N Sore throat Y N Joint swelling Y N Trouble swallowing Y N Myalgias Y N Voice Change Y N Skin Eyes Color change Y N Eye Discharge Y N Pallor Y N Eye itching Y N Rash Y N Eye pain Y N Wound Y N Last Name: ___________________________________ First Name: ______________________________________ Date of Birth: _____________________________ Today’s Date: __________________________________________ Bronson HealthCare Midwest Epic Review of Systems 10.3 Eye redness Y N Allergy/Immuno Photophobia Y N Env allergies Y N Visual disturbance Y N Food Allergies Y N Respiratory Immunocompromised Y N Apnea Y N Neurological Chest tightness Y N Dizziness Y N Choking Y N Facial asymmetry Y N Cough Y N Headaches Y N Shortness of breath Y N Light-headedness -

W10: Causes and Co-Morbidities of Nocturia Workshop Chair: An-Sofie Goessaert, Belgium 12 September 2017 09:00 - 10:30
W10: Causes and Co-morbidities of Nocturia Workshop Chair: An-Sofie Goessaert, Belgium 12 September 2017 09:00 - 10:30 Start End Topic Speakers 09:00 09:20 Phenotyping Nocturia – Judge a Book by its Cover? An-Sofie Goessaert 09:20 09:40 Sleep and Nocturia – Central Mechanisms into Business? Karlien Dhondt 09:40 10:00 Bladder and Kidney – Making the Bladder Gladder or Lowering Philip Van Kerrebroeck the Water Levels? 10:00 10:20 Questionnaire on Nocturia – to TANGO or Not to TANGO? Wendy Bower 10:20 10:30 Questions All Speaker Powerpoint Slides Please note that where authorised by the speaker all PowerPoint slides presented at the workshop will be made available after the meeting via the ICS website www.ics.org/2017/programme Please do not film or photograph the slides during the workshop as this is distracting for the speakers. Aims of Workshop Nocturia is a highly prevalent condition affecting both men and women of all ages. It is no longer a problem merely attributed to overactive bladder or benign prostate hyperplasia. There can be an impairment in one or more factors of the triad brain-kidney- bladder but also other factors such as obesity, hypertension, peripheral edema, sleep disturbance, depression, medication, etc can play a role. The objective of this workshop is to provide an overview on causes and co-morbidities of nocturia and how to identify them. Learning Objectives This workshop should allow the attendant to know the answers to following questions: 1. What physical features can help you to identify possible causes or co-morbidities of nocturia? 2. -

Diabetes - High Blood Sugar After Hours Telephone Triage Protocols | Adult | 2019
Diabetes - High Blood Sugar After Hours Telephone Triage Protocols | Adult | 2019 DEFINITION ⦁ Patient with known diabetes mellitus ⦁ Has a high blood sugar (hyperglycemia), defined as a blood glucose > 200 mg/dL (11 mmol/L) ⦁ Has symptoms of high blood sugar ⦁ Has questions regarding high blood sugar SYMPTOMS of High Blood Sugar (Hyperglycemia) include: ⦁ Mild hyperglycemia: Most often patient will have no symptoms. ⦁ Moderate hyperglycemia: polyuria, polydipsia, fatigue, blurred vision. ⦁ Severe hyperglycemia: confusion and coma. ⦁ Diabetic ketoacidosis (DKA): fruity odor on breath, vomiting, rapid breathing, weakness, confusion, and coma. INITIAL ASSESSMENT QUESTIONS 1. BLOOD GLUCOSE: "What is your blood glucose level?" 2. ONSET: "When did you check the blood glucose?" 3. USUAL RANGE: "What is your glucose level usually?" (e.g., usual fasting morning value, usual evening value) 4. KETONES: "Do you check for ketones (urine or blood test strips)?" If yes, ask: "What does the test show now?" 5. TYPE 1 or 2: "Do you know what type of diabetes you have?" (e.g., Type 1, Type 2, Gestational; doesn't know) 6. INSULIN: "Do you take insulin?" "What type of insulin(s) do you use? What is the mode of delivery? (syringe, pen (e.g., injection or pump) 7. DIABETES PILLS: "Do you take any pills for your diabetes?" If yes, ask: "Have you missed taking any pills recently?" 8. OTHER SYMPTOMS: "Do you have any symptoms?" (e.g., fever, frequent urination, difficulty breathing, dizziness, weakness, vomiting) 9. PREGNANCY: "Is there any chance you are pregnant?" -

A 27-Month-Old Boy with Polyuria and Polydipsia
UC Davis UC Davis Previously Published Works Title A 27-Month-Old Boy with Polyuria and Polydipsia. Permalink https://escholarship.org/uc/item/8x24x4p2 Authors Lee, Yvonne Winnicki, Erica Butani, Lavjay et al. Publication Date 2018 DOI 10.1155/2018/4281217 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Hindawi Case Reports in Pediatrics Volume 2018, Article ID 4281217, 4 pages https://doi.org/10.1155/2018/4281217 Case Report A 27-Month-Old Boy with Polyuria and Polydipsia Yvonne Lee,1 Erica Winnicki,2 Lavjay Butani ,3 and Stephanie Nguyen 3 1Department of Pediatrics, Section of Endocrinology, Kaiser Permanente Oakland Medical Center, Oakland, CA, USA 2Department of Pediatrics, Section of Nephrology, University of California, San Francisco, San Francisco, CA, USA 3Department of Pediatrics, Section of Nephrology, University of California, Davis, Sacramento, CA, USA Correspondence should be addressed to Stephanie Nguyen; [email protected] Received 16 May 2018; Accepted 1 August 2018; Published 23 August 2018 Academic Editor: Anselm Chi-wai Lee Copyright © 2018 Yvonne Lee et al. )is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Psychogenic polydipsia is a well-described phenomenon in those with a diagnosed psychiatric disorder such as schizophrenia and anxiety disorders. Primary polydipsia is differentiated from psychogenic polydipsia by the lack of a clear psychotic disturbance. We present a case of a 27-month-old boy who presented with polyuria and polydipsia. Laboratory studies, imaging, and an observed water deprivation test were consistent with primary polydipsia. -

GESTATIONAL DIABETES TESTING and TREAMENT Accepted: August 2015 Updated: August 2015
Boston Medical Center Maternity Care Guideline: GESTATIONAL DIABETES TESTING AND TREAMENT Accepted: August 2015 Updated: August 2015 INTRODUCTION: Definition: Gestational Diabetes (GDM) is impaired glucose tolerance with first onset or recognition during pregnancy. Prevalence: 4.6-9.2% of US pregnancies are affected by GDM. An increased prevalence of GDM is found among Hispanic, African American, Native American, Asian, and Pacific Islander women.1 Maternal Risk factors for GDM development include: o Personal history of GDM o Obesity (BMI≥30) o PCOS o Impaired glucose tolerance o Glycosuria early in pregnancy o Strong family history of diabetes (one first degree relative, or more than one second degree relative) o Previous macrosomic infant. o Previous unexplained third trimester loss or neonatal death. Maternal/fetal risks of GDM diagnosis: GDM is associated with significant maternal and neonatal morbidity. Maternal risks include development of hypertensive disorders and preeclampsia and development of type 2 diabetes mellitus later in life (50% develop DM within 20years, 60% of Latinas within 5years postpartum).1 Neonatal risks include large for gestational age, macrosomia, shoulder dystocia. , stillbirth, and newborn morbidity including hypoglycemia and respiratory distress. Obstetrical risks include risks associated with macrosomia such as increased rate of operative vaginal birth and cesarean section, brachial plexus injury, fracture, and neonatal depression. DIAGNOSIS: All pregnant women will be screened for gestational diabetes, with the exception of women who already carry a diagnosis of type 1 or type 2 diabetes. The diagnosis will be based on specific criteria. Early Screening for High Risk Populations: Pregnant women who meet the following criteria should be screened as early as possible, preferably at the first prenatal visit. -

Assessing Blood Glucose Regulation
Functional Medicine University’s Functional Diagnostic Medicine Training Program Mod 4 * FDMT533B Primary and Advanced Testing: Assessing Blood Glucose Regulation By Wayne L. Sodano, D.C., D.A.B.C.I., & Ron Grisanti, D.C., D.A.B.C.O., M.S. http://www.FunctionalMedicineUniversity.com Limits of Liability & Disclaimer of Warranty We have designed this book to provide information in regard to the subject matter covered. It is made available with the understanding that the authors are not liable for the misconceptions or misuse of information provided. The purpose of this book is to educate. It is not meant to be a comprehensive source for the topic covered, and is not intended as a substitute for medical diagnosis or treatment, or intended as a substitute for medical counseling. Information contained in this book should not be construed as a claim or representation that any treatment, process or interpretation mentioned constitutes a cure, palliative, or ameliorative. The information covered is intended to supplement the practitioner’s knowledge of their patient. It should be considered as adjunctive and support to other diagnostic medical procedures. This material contains elements protected under International and Federal Copyright laws and treaties. Any unauthorized reprint or use of this material is prohibited. Functional Medicine University; Functional Diagnostic Medicine Training Program/Insider’s Guide Module 4: FDMT 533B: Primary and Advanced Testing: Assessing Blood Glucose Regulation Copyright © 2010 Functional Medicine University, All Rights Reserved Functional Medicine University’s Functional Diagnostic Medicine Training Program Module 4: FDMT 533B: Primary and Advanced Testing: Assessing Blood Glucose Regulation By Wayne L. -
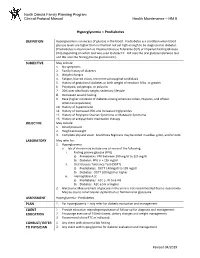
Hyperglycemia – Prediabetes
North Dakota Family Planning Program Clinical Protocol Manual Health Maintenance – HM 8 Hyperglycemia – Prediabetes DEFINITION Hyperglycemia is an excess of glucose in the blood. Prediabetes is a condition when blood glucose levels are higher than normal but not yet high enough to be diagnosed as diabetes. (Prediabetes is also known as Impaired Glucose Tolerance (IGT) or Impaired Fasting Glucose (IFG) depending on which test was used to detect it. IGT uses the oral glucose tolerance test and IFG uses the fasting plasma glucose test.) SUBJECTIVE May include: 1. No symptoms 2. Family history of diabetes 3. Weight changes 4. Fatigue, blurred vision, recurrent vulvovaginal candidiasis 5. History of gestational diabetes or birth weight of newborn 9 lbs. or greater 6. Polydipsia, polyphagia, or polyuria 7. 20% over ideal body weight; sedentary lifestyle 8. Decreased wound healing 9. Race (higher incidence of diabetes among American Indian, Hispanic, and African American population) 10. History of hypertension 11. History of decreased HDL and increased triglycerides 12. History of Polycystic Ovarian Syndrome or Metabolic Syndrome 13. History of antipsychotic medication therapy OBJECTIVE May include: 1. Blood pressure 2. Height and weight 3. Complete physical exam. Acanthosis Nigricans may be noted in axillae, groin, and/or neck LABORATORY May refer for: 1. Hyperglycemia a. lab of choice may include one or more of the following: i. Fasting plasma glucose (FPG) a) Prediabetes: FPG between 100 mg/dl to 125 mg/dl b) Diabetes: FPG is > 126 mg/dl ii. Oral Glucose Tolerance Test (OGTT) a) Prediabetes: OGTT 140mg/dl to 199 mg/dl b) Diabetes: OGTT 200mg/dl or higher iii. -

Guidance on the Clinical Management of Acute and Chronic Harms of Club Drugs and Novel Psychoactive Substances NEPTUNE
Novel Psychoactive Treatment UK Network NEPTUNE Guidance on the Clinical Management of Acute and Chronic Harms of Club Drugs and Novel Psychoactive Substances NEPTUNE This publication of the Novel Psychoactive Treatment UK Network (NEPTUNE) is protected by copyright. The reproduction of NEPTUNE guidance is authorised, provided the source is acknowledged. © 2015 NEPTUNE (Novel Psychoactive Treatment UK Network) 2015 Club Drug Clinic/CAPS Central and North West London NHS Foundation Trust (CNWL) 69 Warwick Road Earls Court SW5 9HB http://www.Neptune-clinical-guidance.com http://www.Neptune-clinical-guidance.co.uk The guidance is based on a combination of literature review and expert clinical con sensus and is based on information available up to March 2015. We accept no responsi bility or liability for any consequences arising from the use of the information contained in this document. The recommended citation of this document is: Abdulrahim D & Bowden-Jones O, on behalf of the NEPTUNE Expert Group. Guidance on the Management of Acute and Chronic Harms of Club Drugs and Novel Psychoactive Substances. Novel Psychoactive Treatment UK Network (NEPTUNE). London, 2015. NEPTUNE is funded by the Health Foundation, an independent charity working to improve the quality of health care in the UK. Editorial production and page design by Ralph Footring Ltd, http://www.footring.co.uk NEPTUNE NEPTUNE (Novel Psychoactive Treatment UK Network): Expert Group members NEPTUNE Expert Group Dr Owen Bowden-Jones Neptune Chair Clinical and programme lead Consultant -

1,5 Anhydroglucitol in Gestational Diabetes Mellitus
Journal of Diabetes and Its Complications 33 (2019) 231–235 Contents lists available at ScienceDirect Journal of Diabetes and Its Complications journal homepage: WWW.JDCJOURNAL.COM 1,5 Anhydroglucitol in gestational diabetes mellitus Thyparambil Aravindakshan Pramodkumar a, Ramamoorthy Jayashri a, Kuppan Gokulakrishnan a, Kaliyaperumal Velmurugan a, Rajendra Pradeepa a, Ulagamathesan Venkatesan a, Ponnusamy Saravanan b, Ram Uma c, Ranjit Mohan Anjana a, Viswanathan Mohan a,⁎ a Madras Diabetes Research Foundation & Dr. Mohan's Diabetes Specialities Centre, WHO Collaborating Centre for Non-communicable Diseases Prevention and Control, ICMR Centre for Advanced Research on Diabetes, Gopalapuram, Chennai, India b Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, United Kingdom c Department of Obstetrics and Gynecology, Seethapathy Clinic and Hospital, Chennai, Tamil Nadu, India article info abstract Article history: Objective: 1,5 Anhydroglucitol (1,5 AG) is reported to be a more sensitive marker of glucose variability and short- Received 25 April 2018 term glycemic control (1–2 weeks) in patients with type1 and type 2 diabetes. However, the role of 1,5 AG in ges- Received in revised form 17 October 2018 tational diabetes mellitus (GDM) is not clear. We estimated the serum levels of 1,5 AG in pregnant women with Accepted 28 November 2018 and without GDM. Available online 5 December 2018 Methods: We recruited 220 pregnant women, 145 without and 75 with GDM visiting antenatal clinics in Tamil Nadu in South India. Oral glucose tolerance tests (OGTTs) were carried out using 82.5 g oral glucose (equivalent Keywords: to 75 g of anhydrous glucose) and GDM was diagnosed based on the International Association of Diabetes and 1,5 Anhydroglucitol Gestational diabetes mellitus Pregnancy Study Group criteria. -

Pheochromocytoma Presenting with Polydipsia And
OLGU SUNUMLARI (Case Reports) PHEOCHROMOCYTOMA PRESENTING WITH POLYDIPSIA AND POLYURIA IN A CHILD Poliüri ve polidipsi ile gelen feokromasitomalý çocuk: Olgu sunumu Ali Baykan1, Nazmi Narin1, Mustafa Kendirci1, Mustafa Akcakus1, Mustafa Küçükaydýn2, Tahir Patýroðlu3 Abstract : Pheochromocytomas are rare tumors in childhood Özet : Feokromositoma çocukluk çaðýnda nadir görülen and can mimic many unrelated diseases due to their various tümörlerdendir ve farklý semptomlarý ile birçok hastalýðý presenting symptoms. While hypertension is the most taklit edebilir. Hipertansiyon feokromositomada en sýk bulgu prevalent finding of pheochromocytomas, polyuria and olmasýna raðmen, poliüri-polidipsi nadir ve ilginç polydipsia are rare and interesting symptoms. In this study we presented a child with unilateral pheochromocytoma, semptomlardýr. Bu yazýda tek taraflý feokromositomalý, ilk whose first symptoms were polyuria-polydipsia, and semptomu poliüri-polidipsi olan ve fizik muayenede hypertension, which were important clues for hipertansiyon tespit edilen vaka takdim edilmiþtir. Klinik ve pheochromocytoma. With the help of clinical and laboratory laboratuar bulgularý ile taný konulan olguda cerrahi findings, patient was diagnosed as pheochromocytoma and rezeksiyon sonrasý bulgu ve belirtiler kayboldu. Multipl referred to surgery; with the removal of the tumor the symptoms disappeared. When the family members were endokrin neoplazi (MEN) açýsýndan aile bireyleri tarandý screened for multiple endocrine neoplasia (MEN) syndromes, ve kýz kardeþine de feokromositoma tanýsý konularak opere a bilateral pheochromocytoma was diagnosed in his sister edildi. Bu makale ile poliüri ve polidipsinin and she was also operated on immediately. In this article feokromositomanýn ilk semptomlarý olabileceðini, ilk we emphasized that polyuria-polydipsia may be the first muayenede tansiyon ölçülmesinin önemini ve symptoms of pheochromocytoma in children, the importance of blood pressure measurement in initial physical examination feokromositomanýn ailesel olabileceðini vurgulamak istedik. -

Download PDF File
Ginekologia Polska 2018, vol. 89, no. 1, 25–29 Copyright © 2018 Via Medica ORIGINAL PAPER / OBSTETRICS ISSN 0017–0011 DOI: 10.5603/GP.a2018.0005 Does reactive hypoglycemia during the 100 g oral glucose tolerance test adversely affect perinatal outcomes? Ilhan Bahri Delibas1, Sema Tanriverdi2, Bulent Cakmak1 1Department of Obstetrics and Gynecology, Gaziosmanpasa University, Tokat, Turkey 2Neonatalogy Clinic, Merkez Efendi State Hospital, Manisa, Turkey ABSTRACT Objectives: To determine whether pregnant women who have reactive hypoglycemia during the 100 g oral glucose toler- ance test (OGTT) are at an increased risk of poor pregnancy outcomes. Material and methods: We retrospectively analyzed perinatal data from 413 women who underwent a 3 h OGTT at 24–28 weeks of gestation and gave birth in our clinics between January 2012 and December 2014. Results: According to OGTT results, the majority of the subjects were normoglycemic (n = 316, 76.5%), while 49 (11.9%) were diagnosed with gestational diabetes, and 33 (8.0%) had single high glucose values. Reactive hypoglycemia was de- tected in only 15 patients (3.6%). The mean age of the women in the reactive hypoglycemia group was significantly lower than that of the women in the gestational diabetes and single high glucose value groups (26.4 ± 4.4 years, 31.4 ± 5.4 years, and 31.8 ± 4.3 years, respectively; p < 0.05). The newborns of the women in the reactive hypoglycemia group had higher rates of APGAR scores < 7, increased admission to the neonatal intensive care unit (NICU), and lower birth weights compared with the other groups (p < 0.001, p < 0.001, and p = 0.009, respectively). -

Diabetes in Pediatrics: Advances in Treatment and Technologies
7/5/2019 Diabetes in Pediatrics: Advances in Treatment and Technologies Lisa Swartz Topor, MD, MMSc Pediatric Endocrinology July 9, 2019 Objectives • Evaluate children presenting with new onset diabetes mellitus • Describe recent advances in insulin pump therapy • Explore how continuous glucose monitors are used in diabetes management Types of Diabetes Mellitus • Type 1A Immune-mediated • Type 1B Insulin-deficient, not autoimmune • Type 2 Combined insulin resistance and insulin secretory deficiency • Secondary Cystic fibrosis; pancreatitis; endocrinopathies (e.g. Cushing syndrome, acromegaly); drug-induced (e.g. glucocorticoids) • Gestational First recognized/onset during pregnancy • Monogenic Maturity-onset diabetes of the young (MODY); insulin signaling defects; insulin gene mutations 1 7/5/2019 Criteria for Diagnosis of Diabetes Mellitus (Non-Pregnant Individuals) Plasma glucose 200 mg/dl at any time plus classic symptoms such as increased urination (polyuria), increased thirst (polydipsia), and weight loss or Fasting plasma glucose 126 mg/dl (fasting 8 hours after last meal) or 2-hour plasma glucose 200 mg/dl during an oral glucose tolerance test (75 g) or Hemoglobin A1c > 6.5% Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010;33(Supp 1):S62-S69. Type 1 Diabetes in Youth Diagnosis of Type 1 Diabetes • Clinical features • Polydipsia, polyuria, polyphagia, weight loss • Absence of signs of insulin resistance (acanthosis nigricans)* • Age, ethnicity, family history • Glucose/A1C criteria • Presence of antibodies