Clinical Practice Guidelines for Diabetes Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Management of Diabetes Mellitus Standards of Care and Clinical Practice Guidelines
WHO-EM/DIN6/E/G MANAGEMENT OF DIABETES MELLITUS STANDARDS OF CARE AND CLINICAL PRACTICE GUIDELINES Edited by Dr A.A.S. Alwan Regional Adviser, Noncommunicable Diseases WHO Regional Office for the Eastern Mediterranean WHO-EM/DIN6/E/G INTRODUCTION Available data from many countries of the Eastern Mediterranean Region (EMR) indicate that diabetes mellitus has become a problem of great magnitude and a major public health concern. Studies have demonstrated that, in some countries, diabetes affects up to 10% of the population aged 20 years and older. This rate may be doubled if those with impaired glucose tolerance (IGT) are also included. The manifestations of diabetes cause considerable human suffering and enormous economic costs. Both acute and late diabetic complications are commonly encountered. Long-term complications represented by cardiovascular diseases, cerebrovascular accidents, end-stage renal disease, retinopathy and neuropathies are already major causes of morbidity, disability and premature death in countries of this Region. The development of long-term complications is influenced by hyperglycaernia. Poor control of diabetes accelerates their progression. Thus, to prevent complications, good control of diabetes is essential and the management of diabetes should therefore aim to improve glycaemic control beyond that required to control its symptoms. Intensified therapy and maintaining near-normal blood glucose levels can result in considerable reduction in the risk of development of retinopathy, nephropathy and neuropathy. However, despite the high prevalence of diabetes and its complications and the availability of successful prevention strategies, essential health care requirements and facilities for self-care are often inadequate in this Region. Action is needed at all levels of health care and in the various aspects of diabetes care to bridge this gap and to improve health care delivery to people with diabetes. -

Clinical Use of Hemoglobin A1c to Improve Diabetes Management
PRACTICAL POINTERS Clinical Use of Hemoglobin A1c to Improve Diabetes Management Alan M. Delamater, PhD, ABPP or more than 25 years, the hemo- one recent study conducted in Norway6 A1C values. Only 14% of the youths globin A1c (A1C) test has been revealed that the majority (82.6%) of were able to accurately describe the A1C Fthe most widely accepted out- 201 adult patients with type 1 diabetes test. Just 11, 7.8, and 7.8% correctly come measure for evaluating glycemic knew what their last A1C was, and most identified the A1C ranges for good, fair, control in individuals with diabetes. patients (90%) knew what a satisfactory and poor glycemic control, respectively. The test provides an index of a patient’s A1C value would be. But a significant Very few youths (1.6–3.2%) knew the average blood glucose level during the number of patients (42%) reported they blood glucose values corresponding to past 2–3 months1 and is considered to had low knowledge of A1C testing in specific A1C results. Only a small num- be the most objective and reliable general. Furthermore, 25% of patients ber of youths correctly estimated the measure of long-term metabolic con- did not think that treatment intensifica- short- and long-term risks associated trol.2,3 The Diabetes Control and tion should occur at an A1C value of with A1C values of 7 and 12%. In this Complications Trial established that 10%. sample, there was a significant lack of maintaining A1C levels as close as pos- A recent cross-sectional study knowledge concerning the meaning and sible to the normal range results in con- examined the relationship between implications of the A1C test. -

Diabetes - High Blood Sugar After Hours Telephone Triage Protocols | Adult | 2019
Diabetes - High Blood Sugar After Hours Telephone Triage Protocols | Adult | 2019 DEFINITION ⦁ Patient with known diabetes mellitus ⦁ Has a high blood sugar (hyperglycemia), defined as a blood glucose > 200 mg/dL (11 mmol/L) ⦁ Has symptoms of high blood sugar ⦁ Has questions regarding high blood sugar SYMPTOMS of High Blood Sugar (Hyperglycemia) include: ⦁ Mild hyperglycemia: Most often patient will have no symptoms. ⦁ Moderate hyperglycemia: polyuria, polydipsia, fatigue, blurred vision. ⦁ Severe hyperglycemia: confusion and coma. ⦁ Diabetic ketoacidosis (DKA): fruity odor on breath, vomiting, rapid breathing, weakness, confusion, and coma. INITIAL ASSESSMENT QUESTIONS 1. BLOOD GLUCOSE: "What is your blood glucose level?" 2. ONSET: "When did you check the blood glucose?" 3. USUAL RANGE: "What is your glucose level usually?" (e.g., usual fasting morning value, usual evening value) 4. KETONES: "Do you check for ketones (urine or blood test strips)?" If yes, ask: "What does the test show now?" 5. TYPE 1 or 2: "Do you know what type of diabetes you have?" (e.g., Type 1, Type 2, Gestational; doesn't know) 6. INSULIN: "Do you take insulin?" "What type of insulin(s) do you use? What is the mode of delivery? (syringe, pen (e.g., injection or pump) 7. DIABETES PILLS: "Do you take any pills for your diabetes?" If yes, ask: "Have you missed taking any pills recently?" 8. OTHER SYMPTOMS: "Do you have any symptoms?" (e.g., fever, frequent urination, difficulty breathing, dizziness, weakness, vomiting) 9. PREGNANCY: "Is there any chance you are pregnant?" -

Glossary of Technical Terms
THIS DOCUMENT IS IN DRAFT FORM, INCOMPLETE AND SUBJECT TO CHANGE AND THAT THE INFORMATION MUST BE READ IN CONJUNCTION WITH THE SECTION HEADED “WARNING” ON THE COVER OF THIS DOCUMENT. GLOSSARY OF TECHNICAL TERMS This glossary contains explanations of certain technical terms used in this Document in connection with our Company and its business. Such terminology and meanings may not correspond to standard industry meanings or usages of those terms. “acetaminophen” a non-opioid analgesic and antipyretic agent used to treat pain and fever “artificial pancreas” an integrated diabetes management system that tracks blood glucose levels using a continuous glucose monitor and automatically delivers the insulin when needed using an insulin pump according to its control algorithm “ascorbic acid” a potent reducing and antioxidant agent that functions in fighting bacterial infections, in detoxifying reactions, and in the formation of collagen in fibrous tissue, teeth, bones, connective tissue, skin, and capillaries “basal insulin” a small, continuous infusion of background insulin delivered automatically at a programmed rate, all day and night “BG Port” blood glucose strip port, the port that accepts and electrically connects a disposable blood glucose strip to the electronics “BGMS” blood glucose monitoring system “BLE” bluetooth low energy “blood glucose” blood glucose, also referred to as blood sugar, is the amount of glucose in your blood, an indicator of diabetes monitoring “bolus insulin” insulin that is taken to lower abnormally high blood glucose -

GESTATIONAL DIABETES TESTING and TREAMENT Accepted: August 2015 Updated: August 2015
Boston Medical Center Maternity Care Guideline: GESTATIONAL DIABETES TESTING AND TREAMENT Accepted: August 2015 Updated: August 2015 INTRODUCTION: Definition: Gestational Diabetes (GDM) is impaired glucose tolerance with first onset or recognition during pregnancy. Prevalence: 4.6-9.2% of US pregnancies are affected by GDM. An increased prevalence of GDM is found among Hispanic, African American, Native American, Asian, and Pacific Islander women.1 Maternal Risk factors for GDM development include: o Personal history of GDM o Obesity (BMI≥30) o PCOS o Impaired glucose tolerance o Glycosuria early in pregnancy o Strong family history of diabetes (one first degree relative, or more than one second degree relative) o Previous macrosomic infant. o Previous unexplained third trimester loss or neonatal death. Maternal/fetal risks of GDM diagnosis: GDM is associated with significant maternal and neonatal morbidity. Maternal risks include development of hypertensive disorders and preeclampsia and development of type 2 diabetes mellitus later in life (50% develop DM within 20years, 60% of Latinas within 5years postpartum).1 Neonatal risks include large for gestational age, macrosomia, shoulder dystocia. , stillbirth, and newborn morbidity including hypoglycemia and respiratory distress. Obstetrical risks include risks associated with macrosomia such as increased rate of operative vaginal birth and cesarean section, brachial plexus injury, fracture, and neonatal depression. DIAGNOSIS: All pregnant women will be screened for gestational diabetes, with the exception of women who already carry a diagnosis of type 1 or type 2 diabetes. The diagnosis will be based on specific criteria. Early Screening for High Risk Populations: Pregnant women who meet the following criteria should be screened as early as possible, preferably at the first prenatal visit. -

Assessing Blood Glucose Regulation
Functional Medicine University’s Functional Diagnostic Medicine Training Program Mod 4 * FDMT533B Primary and Advanced Testing: Assessing Blood Glucose Regulation By Wayne L. Sodano, D.C., D.A.B.C.I., & Ron Grisanti, D.C., D.A.B.C.O., M.S. http://www.FunctionalMedicineUniversity.com Limits of Liability & Disclaimer of Warranty We have designed this book to provide information in regard to the subject matter covered. It is made available with the understanding that the authors are not liable for the misconceptions or misuse of information provided. The purpose of this book is to educate. It is not meant to be a comprehensive source for the topic covered, and is not intended as a substitute for medical diagnosis or treatment, or intended as a substitute for medical counseling. Information contained in this book should not be construed as a claim or representation that any treatment, process or interpretation mentioned constitutes a cure, palliative, or ameliorative. The information covered is intended to supplement the practitioner’s knowledge of their patient. It should be considered as adjunctive and support to other diagnostic medical procedures. This material contains elements protected under International and Federal Copyright laws and treaties. Any unauthorized reprint or use of this material is prohibited. Functional Medicine University; Functional Diagnostic Medicine Training Program/Insider’s Guide Module 4: FDMT 533B: Primary and Advanced Testing: Assessing Blood Glucose Regulation Copyright © 2010 Functional Medicine University, All Rights Reserved Functional Medicine University’s Functional Diagnostic Medicine Training Program Module 4: FDMT 533B: Primary and Advanced Testing: Assessing Blood Glucose Regulation By Wayne L. -
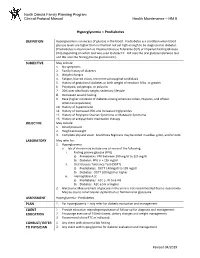
Hyperglycemia – Prediabetes
North Dakota Family Planning Program Clinical Protocol Manual Health Maintenance – HM 8 Hyperglycemia – Prediabetes DEFINITION Hyperglycemia is an excess of glucose in the blood. Prediabetes is a condition when blood glucose levels are higher than normal but not yet high enough to be diagnosed as diabetes. (Prediabetes is also known as Impaired Glucose Tolerance (IGT) or Impaired Fasting Glucose (IFG) depending on which test was used to detect it. IGT uses the oral glucose tolerance test and IFG uses the fasting plasma glucose test.) SUBJECTIVE May include: 1. No symptoms 2. Family history of diabetes 3. Weight changes 4. Fatigue, blurred vision, recurrent vulvovaginal candidiasis 5. History of gestational diabetes or birth weight of newborn 9 lbs. or greater 6. Polydipsia, polyphagia, or polyuria 7. 20% over ideal body weight; sedentary lifestyle 8. Decreased wound healing 9. Race (higher incidence of diabetes among American Indian, Hispanic, and African American population) 10. History of hypertension 11. History of decreased HDL and increased triglycerides 12. History of Polycystic Ovarian Syndrome or Metabolic Syndrome 13. History of antipsychotic medication therapy OBJECTIVE May include: 1. Blood pressure 2. Height and weight 3. Complete physical exam. Acanthosis Nigricans may be noted in axillae, groin, and/or neck LABORATORY May refer for: 1. Hyperglycemia a. lab of choice may include one or more of the following: i. Fasting plasma glucose (FPG) a) Prediabetes: FPG between 100 mg/dl to 125 mg/dl b) Diabetes: FPG is > 126 mg/dl ii. Oral Glucose Tolerance Test (OGTT) a) Prediabetes: OGTT 140mg/dl to 199 mg/dl b) Diabetes: OGTT 200mg/dl or higher iii. -

1,5 Anhydroglucitol in Gestational Diabetes Mellitus
Journal of Diabetes and Its Complications 33 (2019) 231–235 Contents lists available at ScienceDirect Journal of Diabetes and Its Complications journal homepage: WWW.JDCJOURNAL.COM 1,5 Anhydroglucitol in gestational diabetes mellitus Thyparambil Aravindakshan Pramodkumar a, Ramamoorthy Jayashri a, Kuppan Gokulakrishnan a, Kaliyaperumal Velmurugan a, Rajendra Pradeepa a, Ulagamathesan Venkatesan a, Ponnusamy Saravanan b, Ram Uma c, Ranjit Mohan Anjana a, Viswanathan Mohan a,⁎ a Madras Diabetes Research Foundation & Dr. Mohan's Diabetes Specialities Centre, WHO Collaborating Centre for Non-communicable Diseases Prevention and Control, ICMR Centre for Advanced Research on Diabetes, Gopalapuram, Chennai, India b Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, United Kingdom c Department of Obstetrics and Gynecology, Seethapathy Clinic and Hospital, Chennai, Tamil Nadu, India article info abstract Article history: Objective: 1,5 Anhydroglucitol (1,5 AG) is reported to be a more sensitive marker of glucose variability and short- Received 25 April 2018 term glycemic control (1–2 weeks) in patients with type1 and type 2 diabetes. However, the role of 1,5 AG in ges- Received in revised form 17 October 2018 tational diabetes mellitus (GDM) is not clear. We estimated the serum levels of 1,5 AG in pregnant women with Accepted 28 November 2018 and without GDM. Available online 5 December 2018 Methods: We recruited 220 pregnant women, 145 without and 75 with GDM visiting antenatal clinics in Tamil Nadu in South India. Oral glucose tolerance tests (OGTTs) were carried out using 82.5 g oral glucose (equivalent Keywords: to 75 g of anhydrous glucose) and GDM was diagnosed based on the International Association of Diabetes and 1,5 Anhydroglucitol Gestational diabetes mellitus Pregnancy Study Group criteria. -

Diabetes Management: Directory of Provider Resources (PDF)
DIABETES MANAGEMENT: DIRECTORY OF PROVIDER RESOURCES 2 Diabetes Management: Directory of Provider Resources ACKNOWLEDGMENTS Diabetes Management: Directory of Provider Resources was prepared with input from National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases; Centers for Medicare & Medicaid Services Advisory Panel on Outreach and Education; NORC at the University of Chicago; Jamie Murkey, MPH, PhD, Program Alignment and Partner Engagement Group 2019 summer intern; Asian Services in Action – International Community Health Center; Chinatown Public Health Center; Colorado Coalition for the Homeless; Garden City Community Health Center, Genesis Family Health; Jackson Medical Mall and Jackson Hinds Comprehensive Health Center; Montefiore Medical Center; Nash Health Care Systems; Sun Life Family Health Center; and Bryan W. Whitfield Memorial Hospital, Tombigbee Healthcare Authority. 3 Diabetes Management: Directory of Provider Resources PURPOSE The purpose of this directory is to support providers and care teams by identifying resources on the management of type 2 diabetes. It is particularly suited for providers who work with Medicare beneficiaries and vulnerable populations for whom the prevalence of type 2 diabetes and diabetes complications is higher. This directory will help the care team identify resources to improve diabetes management by promoting medication adherence. This directory also aims to equip primary care teams with tools to manage diabetes and that patients with more complex needs are appropriately referred to specialists. While some patients require care from endocrinologists, primary care teams can effectively manage many patients with prediabetes and type 2 diabetes.i Other health professionals and patients can play an important role in facilitating medication management and other diabetes self-care behaviors. -

Download PDF File
Ginekologia Polska 2018, vol. 89, no. 1, 25–29 Copyright © 2018 Via Medica ORIGINAL PAPER / OBSTETRICS ISSN 0017–0011 DOI: 10.5603/GP.a2018.0005 Does reactive hypoglycemia during the 100 g oral glucose tolerance test adversely affect perinatal outcomes? Ilhan Bahri Delibas1, Sema Tanriverdi2, Bulent Cakmak1 1Department of Obstetrics and Gynecology, Gaziosmanpasa University, Tokat, Turkey 2Neonatalogy Clinic, Merkez Efendi State Hospital, Manisa, Turkey ABSTRACT Objectives: To determine whether pregnant women who have reactive hypoglycemia during the 100 g oral glucose toler- ance test (OGTT) are at an increased risk of poor pregnancy outcomes. Material and methods: We retrospectively analyzed perinatal data from 413 women who underwent a 3 h OGTT at 24–28 weeks of gestation and gave birth in our clinics between January 2012 and December 2014. Results: According to OGTT results, the majority of the subjects were normoglycemic (n = 316, 76.5%), while 49 (11.9%) were diagnosed with gestational diabetes, and 33 (8.0%) had single high glucose values. Reactive hypoglycemia was de- tected in only 15 patients (3.6%). The mean age of the women in the reactive hypoglycemia group was significantly lower than that of the women in the gestational diabetes and single high glucose value groups (26.4 ± 4.4 years, 31.4 ± 5.4 years, and 31.8 ± 4.3 years, respectively; p < 0.05). The newborns of the women in the reactive hypoglycemia group had higher rates of APGAR scores < 7, increased admission to the neonatal intensive care unit (NICU), and lower birth weights compared with the other groups (p < 0.001, p < 0.001, and p = 0.009, respectively). -

Diabetes in Pediatrics: Advances in Treatment and Technologies
7/5/2019 Diabetes in Pediatrics: Advances in Treatment and Technologies Lisa Swartz Topor, MD, MMSc Pediatric Endocrinology July 9, 2019 Objectives • Evaluate children presenting with new onset diabetes mellitus • Describe recent advances in insulin pump therapy • Explore how continuous glucose monitors are used in diabetes management Types of Diabetes Mellitus • Type 1A Immune-mediated • Type 1B Insulin-deficient, not autoimmune • Type 2 Combined insulin resistance and insulin secretory deficiency • Secondary Cystic fibrosis; pancreatitis; endocrinopathies (e.g. Cushing syndrome, acromegaly); drug-induced (e.g. glucocorticoids) • Gestational First recognized/onset during pregnancy • Monogenic Maturity-onset diabetes of the young (MODY); insulin signaling defects; insulin gene mutations 1 7/5/2019 Criteria for Diagnosis of Diabetes Mellitus (Non-Pregnant Individuals) Plasma glucose 200 mg/dl at any time plus classic symptoms such as increased urination (polyuria), increased thirst (polydipsia), and weight loss or Fasting plasma glucose 126 mg/dl (fasting 8 hours after last meal) or 2-hour plasma glucose 200 mg/dl during an oral glucose tolerance test (75 g) or Hemoglobin A1c > 6.5% Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010;33(Supp 1):S62-S69. Type 1 Diabetes in Youth Diagnosis of Type 1 Diabetes • Clinical features • Polydipsia, polyuria, polyphagia, weight loss • Absence of signs of insulin resistance (acanthosis nigricans)* • Age, ethnicity, family history • Glucose/A1C criteria • Presence of antibodies -

Delivering on Our Commitment to Patients
Delivering on our commitment to patients Zealand Pharma Corporate Presentation Forward-looking statements This presentation contains information pertaining to Zealand Pharma A/S (“Zealand"). Neither Zealand nor its management, directors, employees or representatives make any representation or warranty, express or implied, as to the accuracy or completeness of any of the information contained in this presentation or any other information transmitted or made available to the viewer or recipient hereof, whether communicated in written or oral form. This presentation does not constitute or form part of, and should not be construed as, an offer to sell or issue or the solicitation of an offer to buy or acquire Zealand securities, in any jurisdiction, or an inducement to enter into investment activity, nor shall there be any sale of Zealand securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. No part of this presentation, nor the fact of its distribution, should form the basis of, or be relied on in connection with, any contract or commitment or investment decision whatsoever. This presentation contains forward-looking statements that reflect management's current views with respect to Zealand's product candidates' development, clinical and regulatory timelines and anticipated results, market opportunity, potential financial performance and other statements of future events or conditions. Although Zealand believes that the expectations reflected in such forward-looking statements are reasonable, no assurance can be given that such expectations will prove to have been correct. Accordingly, results could differ materially from those set out in the forward-looking statements as a result of various factors, many of which are beyond Zealand’s control.