Salmonid Behavior Water Temperature
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stefan Konstańczak Human Ecology
Stefan Konstańczak Human ecology Studia Ecologiae et Bioethicae 4, 9-15 2006 7 " Studia E cologiae et Bioethicae 4/2006 Stefan KONSTAŃCZAK PAP Słupsk Human ecology Introduction Several years ago UNESCO published a document presenting fields of knowledge that will be necessary in future education rendered to societies.1 ^ e document comprises seven chapters, which, among others, deal with the problems pertaining to shaping of group identity of the entire humanity, threats and hopes along the way toward development of the human species, sense of human existence put into individual and social perspectives, and concludes by calling for new ethics that would embrace changes to world order that the present world is currently facing. In this epoch it is human that is the single highest good (summum bonum), and all other values will gain their significance based on their scope of relations with the man. ^ is work’s guiding principle is the concern over the future of human species, which, according to its author is threatened not so much by degradation of nature, as by degradation of social environment. Upon reading that paper one can conclude that men cease to control their artifacts, especially those of science and technology; they are no longer capable of accomplishing their goals in a simple, direct manner. More and more commonly people feel lost and alienated from reality in they live in. It is as if we were to learn anew how to handle everyday life’s situations. At the same time, as Morin declares, it is the humanity that is our planet’s destiny, and it is hard to imagine Earth devoid of our presence anymore. -
Lake Huron Spawning
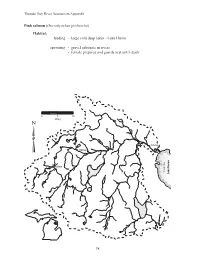
Thunder Bay River Assessment Appendix Pink salmon (Oncorhynchus gorbuscha) Habitat: feeding - large cold deep lakes - Lake Huron spawning - gravel substrate in rivers - female prepares and guards nest until death 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 98 Thunder Bay River Assessment Appendix Coho salmon (Oncorhynchus kisutch) Habitat: feeding - adults: Lake Huron - young: shallow gravel substrate in cold streams, later into pools spawning - cold streams and rivers - swifter water of shallow gravelly substrate 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 99 Thunder Bay River Assessment Appendix Rainbow trout (Oncorhynchus mykiss) Habitat: feeding - cold clear water of rivers and Lake Huron - moderate current spawning - gravelly riffles above a pool - smaller tributaries 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 100 Thunder Bay River Assessment Appendix Chinook salmon (Oncorhynchus tshawyscha) Habitat: feeding - adults: Lake Huron - young: shallow gravel substrate in cool streams, later into pools spawning - gravelly substrate in cool streams 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 101 Thunder Bay River Assessment Appendix Round whitefish (Prosopium cylindraceum) Habitat: feeding - lakes, rivers, and streams spawning - shallows of lakes and rivers - gravel or rock substrate 0 5 10 Miles Alpena Hillman Atlanta Thunder Bay Lake Huron 102 Thunder Bay River Assessment Appendix Atlantic salmon (Salmo salar) Habitat: feeding - young: gravel substrate streams - adults: Lake Huron -

Salmon Fact Sheet
THE WILD SALMON SEAFOOD MARKET’S GUIDE TO W I L D P A C I F I C S A L M O N Salmon Pacific Salmon occur from northern California along the Pacific Coast throughout the Pacific Ocean, Bering Sea and Arctic Ocean waters adjacent to Alaska. Salmon are anadromous, that is, they spawn in fresh water and the young migrate to the sea where they mature. The mature Salmon returns to the stream of their birth to spawn. Nutrition Few single foods bring as many valuable contributions to the table as Salmon. An excellent source of high-quality protein, containing all the essential amino acids. The fats in Salmon are predominately unsaturated. These fats are evidenced to reduce the risk of heart disease. Availability Although each species has a particular season, small fisheries of wild salmon occur periodically, making fresh salmon (often hard to find and expensive) available throughout the year. Your best values will come during peak salmon season, May through September. Frozen salmon (often frozen at sea) is available during the off season. Also known as Chinook Salmon. Also known as Silver Salmon. Highly desired for King The largest of the species and the most Coho both table use and smoking. Coho salmon offers prominent of the salmon known for its high oil firm meat with excellent flavor slightly milder than content and distinctive, rich flavor. King and Sockeye. Average size from 5 to 40 lbs. Average size from 4 - 9 lbs. Available May - September Available June - September Copper River & Yukon River King Also known as Chum Salmon. -

Imagine the Silver Beauty and the Fighting Spirit of Atlantic Salmon; The
Sakhalin Silver Text and Photos: Clemens Ratschan Imagine the silver beauty and the fighting spirit of Atlantic salmon; the complex, unpredictable life- history of sea trout and combine with the ferocious take and body mass of a predatory taimen. This will give you a glimpse of what fishing for Sakhalin taimen, the silver of the Russian Far East, is about. AM PLEASED TO introduce Siberian taimen, Hucho taimen. No this fish to the readers of wonder, scientists also erroneously Chasing Silver, because in related this far-eastern species to many respects it forms a the large-sized, non-anadromous missing link between the predators of the genus Hucho, which Ifishery for anadromous salmon and is a branch of the salmonoid tree for huchen, a big predatory non- that occurs exclusively in Eurasia. anadromous salmonoid in my home In Central Europe, Hucho hucho is country of Austria (‘Danube salmon’ restricted to the Danube System, in English. See article “Taimen” by where self-sustaining stocks are Wolfgang Hauer, issue 3/2010). presently only found in a handful of Sakhalin taimen is one of the rivers in Germany, Austria, Slovakia least-known salmonid species among and former Yugoslavia. Huchen is non-Russian fishermen; even many very closely related to the already- Russians tend to confuse it with the mentioned Siberian taimen. The latter | 62 | Chasing Silver Fly Fishing Magazine April’s Fav Five www.chasingsilvermagazine.com | 63 | Sakhalin Silver inhabits a distant, vast range from a habits. But one ecological feature expeditions to Japan. Later, the fish few places in European Russia to the is unique – all members of the true was assigned to the genus Parahucho, Lena and Amur rivers in the very far huchen live exclusively in fresh water, with regard to some obvious east of northern Asia. -

Full Text in Pdf Format
Vol. 27: 277–287, 2015 ENDANGERED SPECIES RESEARCH Published online May 13 doi: 10.3354/esr00675 Endang Species Res OPENPEN ACCESSCCESS Causes of the drastic loss of genetic variation in the Critically Endangered Formosa landlocked salmon of Taiwan Te-Hua Hsu1, Keisuke Takata2, Hiroshi Onozato3, Jin-Chywan Gwo1,* 1Department of Aquaculture, National Taiwan Ocean University, Keelung 20224, Taiwan 2Faculty of Science, Shinshu University, Matsumoto-city, Nagano 390-8621, Japan 3Matsumoto Institute of Microorganisms Co. Ltd., Matsumoto-city, Nagano 390-1241, Japan ABSTRACT: The use of hatchery-reared fish to replenish existing threatened wild populations has been shown to reduce or change the natural genetic diversity of the wild populations. In this study, the genetic diversity of wild Formosa landlocked salmon Oncorhynchus formosanus in its main habitat of the Chichiawan Stream in Taiwan was examined after a large-scale escape of hatchery- cultivated fish. Approximately 3000 individuals (the descendants of only 5 pairs of wild salmon) es- caped from an old hatchery when Typhoon Ariel breached the hatchery in the fall of 2004. The ge- netic diversity of the wild population was extremely low at that time, and declined further between 2004 and 2008 following the escape of hatchery fish. We hypothesize that the decline in genetic di- versity of the wild population was mainly caused by a population bottleneck in 2005, and that ge- netic homogeneity since 2005 was caused by breeding of the escaped hatchery fish (which showed low genetic diversity) that survived the floods of 2004. This supports the possibility that the drastic decline in genetic diversity between 2004 and 2008 was caused by the genetic effects of the escaped hatchery fish, and demonstrates the risk of introducing hatchery fish into the wild. -

Evaluating Juvenile Thermal Tolerance As a Constraint on Adult Range of Gray Snapper (Lutjanus Griseus): a Combined Laboratory, Field and Modeling Approach
Evaluating juvenile thermal tolerance as a constraint on adult range of gray snapper (Lutjanus griseus): A combined laboratory, field and modeling approach Mark J. Wuenschel, Jonathan A. Hare, Matthew E. Kimball, and Kenneth W. Able SEDAR51-RD-17 October 2016 Journal of Experimental Marine Biology and Ecology 436-437 (2012) 19–27 Contents lists available at SciVerse ScienceDirect Journal of Experimental Marine Biology and Ecology journal homepage: www.elsevier.com/locate/jembe Evaluating juvenile thermal tolerance as a constraint on adult range of gray snapper (Lutjanus griseus): A combined laboratory, field and modeling approach Mark J. Wuenschel a,b,⁎, Jonathan A. Hare c, Matthew E. Kimball a,d,e, Kenneth W. Able a a Marine Field Station, Institute of Marine and Coastal Sciences, Rutgers University, 800 c/o 132 Great Bay Boulevard, Tuckerton, NJ 08087, United States b NOAA NMFS Woods Hole Laboratory, 166 Water Street, Woods Hole, MA 02543, United States c NOAA NMFS Narragansett Laboratory, 28 Tarzwell Drive, Narragansett, RI 02882, United States d GTM National Estuarine Research Reserve, 505 Guana River Road, Ponte Vedra Beach, FL 32082, United States e Biology Department, University of North Florida, 1 UNF Drive, Jacksonville, FL 32224, United States article info abstract Article history: Climate change is expected to cause a poleward shift of many temperate species, however, a mechanistic un- Received 6 December 2011 derstanding of how temperature and species' life histories interact to produce observed adult range is often Received in revised form 15 August 2012 lacking. We evaluated the hypothesis that juvenile thermal tolerance determines northern range in gray Accepted 16 August 2012 snapper (Lutjanus griseus), a species commonly caught as juveniles along the US Atlantic coast well north Available online xxxx of their adult distribution, using a combined laboratory, field and modeling approach. -

Adverse Outcome Pathways and Ecological Risk Assessment: Bridging to Population-Level Effects
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Publications, Agencies and Staff of the U.S. Department of Commerce U.S. Department of Commerce 2010 ADVERSE OUTCOME PATHWAYS AND ECOLOGICAL RISK ASSESSMENT: BRIDGING TO POPULATION-LEVEL EFFECTS Vincent J. Kramer Dow AgroSciences, [email protected] Matthew A. Etterson U.S. Environmental Protection Agency Markus Hecker ENTRIX Cheryl A. Murphy Michigan State University Guritno Roesijadi Pacific Northwest National Laboratory See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/usdeptcommercepub Part of the Environmental Sciences Commons Kramer, Vincent J.; Etterson, Matthew A.; Hecker, Markus; Murphy, Cheryl A.; Roesijadi, Guritno; Spade, Daniel J.; Spromberg, Julann A.; Wang, Magnus; and Ankley, Gerald T., "ADVERSE OUTCOME PATHWAYS AND ECOLOGICAL RISK ASSESSMENT: BRIDGING TO POPULATION-LEVEL EFFECTS" (2010). Publications, Agencies and Staff of the U.S. Department of Commerce. 217. https://digitalcommons.unl.edu/usdeptcommercepub/217 This Article is brought to you for free and open access by the U.S. Department of Commerce at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Publications, Agencies and Staff of the U.S. Department of Commerce by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Vincent J. Kramer, Matthew A. Etterson, Markus Hecker, Cheryl A. Murphy, Guritno Roesijadi, Daniel J. Spade, Julann A. Spromberg, Magnus Wang, and Gerald T. Ankley This article is available at DigitalCommons@University of Nebraska - Lincoln: https://digitalcommons.unl.edu/ usdeptcommercepub/217 Environmental Toxicology and Chemistry, Vol. 30, No. 1, pp. 64–76, 2011 # 2010 SETAC Printed in the USA DOI: 10.1002/etc.375 Predictive Ecotoxicology Workshop ADVERSE OUTCOME PATHWAYS AND ECOLOGICAL RISK ASSESSMENT: BRIDGING TO POPULATION-LEVEL EFFECTS VINCENT J. -

Variation in Salmonid Life Histories: Patterns and Perspectives
United States Department of Agriculture Variation in Salmonid Life Forest Service Histories: Patterns and Pacific Northwest Research Station Perspectives Research Paper PNW-RP-498 Mary F. Willson February 1997 Author MARY F. WILLSON is a research ecologist, Forestry Sciences Laboratory, 2770 Sherwood Lane, Juneau, AK 98801. Abstract Willson, Mary F. 1997. Variation in salmonid life histories: patterns and perspectives. Res. Pap. PNW-RP-498. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 50 p. Salmonid fishes differ in degree of anadromy, age of maturation, frequency of repro- duction, body size and fecundity, sexual dimorphism, breeding season, morphology, and, to a lesser degree, parental care. Patterns of variation and their possible signif- icance for ecology and evolution and for resource management are the focus of this review. Keywords: Salmon, char, Oncorhynchus, Salmo, Salvelinus, life history, sexual dimor- phism, age of maturation, semelparity, anadromy, phenology, phenotypic variation, parental care, speciation. Summary Salmonid fishes differ in degree of anadromy, age of maturation, frequency of reproduction, body size and fecundity, sexual dimorphism, breeding season, morphology, and to a lesser degree, parental care. The advantages of large body size in reproductive competition probably favored the evolution of ocean foraging, and the advantages of safe breeding sites probably favored freshwater spawning. Both long-distance migrations and reproductive competition may have favored the evolution of semelparity. Reproductive competition has favored the evolution of secondary sexual characters, alternative mating tactics, and probably nest-defense behavior. Salmonids provide good examples of character divergence in response to ecological release and of parallel evolution. The great phenotypic plasticity of these fishes may facilitate speciation. -

Attention California Ocean Salmon Anglers
Attention California Ocean Salmon Anglers Please check your catch! Coho salmon are frequently contacted in California’s ocean fisheries. Although some of these salmon may have originated from Oregon or Washington, many are California coastal coho salmon, which are protected under the Endangered Species Act. Thus the retention of coho salmon is PROHIBITED in all California ocean fisheries. Please take the time to correctly identify each salmon caught before removing it from the water. All coho must be released. *Photo by CDFW Warden Bob Aldrich Help avoid contacting coho salmon: Rig to fish deeper - coho are more often in the top 30 feet of water. Fish nearshore for Chinook - coho are usually more offshore. Use large lures that select for larger Chinook and reduce coho catch. For additional information, please check the CDFW website at www.dfg.ca.gov/marine/oceansalmon.asp or call the Ocean Salmon Hotline at (707) 576-3429 Note: A few pink salmon have been caught in past seasons, usually in odd numbered years. Pink salmon are generally smaller than Chinook and coho salmon and can be identified by the large, oval- shaped spots found on their back and on both lobes of the tail fin. Their scales are very small and number over 168 in the row above the lateral line. The minimum size limit in California for pink salmon is the same as Chinook. The daily bag/possession limit remains 2 salmon of any species except coho. . -

HOW to IDENTIFY the FIVE SALMON SPECIES Found in the KODIAK ISLAND/ALASKA PENINSULA AREA
HOW TO IDENTIFY the FIVE SALMON SPECIES found in the KODIAK ISLAND/ALASKA PENINSULA AREA KING (CHINOOK) SALMON: COHO (SILVER) SALMON: Greenish-blue Blue-gray back with silvery sides. Small, irregular- back with silvery sides. Small black spots on the back, shaped black spots on back, dorsal fi n, and usually on dorsal fi n, and both lobes of the tail. usually on Black mouth with white upper lobe gums at base of teeth on of tail lower jaw. only. Spawning coho salmon adults develop Black mouth with black gums greenish-black heads and dark brown to at base of teeth on lower jaw. maroon bodies. Salmon mouth illustrations courtesy of California Department Fish and Game SOCKEYE (RED) SALMON: Dark blue-black back with silvery sides. No distinct Spawning spots on back, king salmon dorsal fi n, adults lose their or tail. silvery bright color and take Spawning sockeye salmon adults develop dull on a maroon to olive brown color. green colored heads and brick-red to scarlet bodies. CHUM (DOG) SALMON: Dull gray back PINK SALMON (HUMPIES): with yellowish-silver sides. Very large spots on the back No distinct spots on back and large black oval blotches or tail. Large eye pupil— on both tail lobes. Very small covers nearly the entire eye. scales. Spawning adults take on a dull gray coloration on the back and Spawning adults develop olive green upper sides with a creamy white coloration on the back with maroon color below. Males develop a sides covered with irregular dull red pronounced hump. bars. Males exhibit many large canine- like teeth. -

Waters Are More Polluted Than Tests Say: Standard
Q: For the benefit of our readers, could you give a bit of information on your academic background, areas of interest, and tell us why you chose to carry out this study? A: Currently I’m a post-doctoral scholar at Dr Richard Connon’s lab at WATERS ARE MORE POLLUTED the Anatomy, Physiology and Cell Biology Dept. at The University of California Davis. I got my PHD degree from the Technical University of Munich (TUM) in Germany, but I conducted all my research for my previous studies at the University of California Davis, in collaboration THAN TESTS SAY: with TUM. I have been in the US since 2010, and I’ve been working mainly on the effects of pesticide mixtures on the aquatic environment. My main focus currently is on the invertebrate species STANDARD TOXICITY that all fish and higher trophic organisms need - I am convinced that if we know what is going on at the lower end of the food chain that we can help all the other organisms throughout the chain. ANALYSES COME UP SHORT Before I came to the US I earned my Masters degree at TUM in Ecotoxicology, Limnology and Ecology, and I’d also already studied the effects of pesticides on invertebrate communities. Currently I’m looking at contaminants of all different kinds –insecticides, herbicides, AN INTERVIEW WITH DR. SIMONE HASENBEIN pharmaceuticals – and what their effects are on invertebrate species, as well as algae species, in lab toxicity studies and field studies. We recently found that contaminants are no longer found in such high concentrations out in the field so as to cause mortality, so we shifted our focus to sublethal end points. -

Dissertation (2.087Mb)
ABSTRACT Advancing an Understanding of Ecological Risk Assessment Approaches for Ionizable Contaminants in Aquatic Systems. Theodore W. Valenti Jr., Ph.D. Committee Chairperson: Bryan W. Brooks, Ph.D. Freshwater is increasingly becoming a finite resource in many regions of the world. Gaps between estimated water supply and demand continue to narrow and the prospects of acquiring additional sources of freshwater remain limited. Furthermore, economically efficient water resource management practices are perplexed by increasing urbanization and changing land-use in semi-arid regions. Although repeated use of water is a practical and effective means for easing strain on water supplies, there is concern that unnecessary contamination may diminish future value of this important resource. Some surface waters in semi-arid regions of the U.S. are effluent-dominated as flow is comprised of >90% treated wastewater. Ionizable compounds are chemicals often associated with urban development and examples include pharmaceuticals, agrochemicals, natural toxins, and other common contaminants (e.g. ammonia). Because continued population growth and urbanization are likely to increase contaminant release and alter dilution capacity of receiving systems, it is important that best management approaches are developed at the watershed scale to limit water quality degradation associated with ionizable compounds. Current methods for prospective and retrospective ecological risk assessments of ionizable compounds seldom consider site-specific conditions during the analysis of effects of phase. Ionization state is largely controlled by the acid/base dissociation constant (pKa) and pH of the solution where a compound resides. Stream water quality can therefore influence ionization state, which is important because the unionized forms a more lipophilic and have a greater propensity to cross cellular membranes.