2020 VQI Year in Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

March 6, 2019 – March 12, 2019 Need Projection Analyses
Use the links below for easy navigation Letters of Intent Letters of Intent - Expired New CON Applications Pending/Complete Applications Pending Review/Incomplete CON Applications Office of Health Planning Recently Approved CON Applications Recently Denied CON Applications Appealed CON Projects Letters of Determination Requests for Miscellaneous Letters of Determination Appealed Determinations DET Review LNR Conversion Requests for LNR for Diagnostic or Therapeutic Equipment Requests for LNR for Establishment CERTIFICATE OF NEED of Physician-Owned Ambulatory Surgery Facilities Appealed LNRs Requests for Extended Implementation/Performance Period Batching Notifications - Fall March 6, 2019 – March 12, 2019 Need Projection Analyses New Batching Review Winter Cycle Fall Cycle Non-Filed or Incomplete Surveys Georgia Department of Community Health Office of Health Planning Indigent-Charity Shortfalls 2 Peachtree Street 5th Floor CON Filing Requirements (effective July 18, 2017) Atlanta, Georgia 30303-3159 Contact Information (404) 656-0409 (404) 656-0442 Fax Verification of Lawful Presence within U.S. www.dch.georgia.gov Periodic Reporting Requirements CON Thresholds Open Record Request Form Web Links Certificate of Need Appeal Panel www.GaMap2Care.info Letters of Intent LOI2019010 Tanner Imaging Center, Inc. Development of Freestanding Imaging Center on Tanner Medical Center-Carrollton Campus Received: 2/15/2019 Application must be submitted on: 3/18/2019 Site: 706 Dixie Street, Carrollton, GA 30117 (Carroll County) Estimated Cost: $2,200,000 -

2019 Cardiovascular Update May 31, 2019
2019 Cardiovascular Update May 31, 2019 Drew Lecture Hall Medical Services Building Piedmont Athens Regional Athens, GA About the Conference Welcome The Georgia Chapter of the American College of Cardiology (ACC) would like to invite you to attend the 2019 Car- diovascular Update. This meeting reflects our dedication to reducing cardiovascular risk and improving patient outcomes. Registered Nurses, Nurse Practitioners, Clinical Nurse Specialists, Physician Assistants, and Clinical Pharmacists should participate in order to enhance their knowledge, improve assessment skills, and network with colleagues. At this meeting, you will have the opportunity to learn more about the latest research and clinical guideline updates in cardiovascular medicine and apply this information to your own clinical practice. We look forward to providing you with an individualized experience that meets all of your learning needs. Kathi Davis, RN, CCCC Program Director About the Conference Parking The conference registration desk will be open from The conference will be held in the Drew 7:00am - 3:00pm on Friday. The program will begin Lecture Hall which is located on the third floor of the promptly at 8:00am and continue until 3:00pm. Medical Services Building (#8 on the map below). You may park in the MSB parking lot and one of the CME All registrants will need to provide their member or non team will validate your parking ticket if needed. member ID# and email address. Each attendee will be sent an evaluation link from [email protected] following the course. Once the evaluation has been completed, you will be prompted to enter your name (as you would like for it to appear on your certificate) and your email address. -

HB 789 Surprise Bill Transparency Act Information
HB 789 Surprise Bill Transparency Act Information As part of the HB 789 Surprise Bill Transparency Act, Ambetter from Peach State Health Plan is providing consumers with the surprise bill rating for in-network hospital specialty groups. If a surprise bill rating is less than four checkmarks, each insurer advertising a hospital as in-network shall describe which qualified hospital based specialty group types are not contracted with the insurer. Please use the legend below to determine the rating for in-hospital specialty groups. Key Service Provided by In-Network Provider Service Provided by Out of Network Provider Service not offered at Facility Specialty Network Participation in Review To view a full listing of our in-network provider’s visit: https://guide.ambetterhealth.com/ NOTE: The information in this document was accurate when published. Since then, changes may have occurred that affect the information. Please call Member Services at 1-877-687-1180 or TTY/TDD 1-877-941-9231 for the most current provider information. 10/2020 This document is current as of the date listed below. A provider’s listing in the directory does not guarantee that the provider is still in the network or accepting new members. Ambetter from Peach State Health Plan 10/2020 TABLE OF CONTENTS / TABLA DE CONTENIDOS Page / Página Hospitals / Hospitales .......................................................................... 2 Index / Índice ........................................................................................ 6 Peach State Health Plan November/2020 -

2018 Participating Organizations
2018 Participating Organizations 3M Company Albert Einstein College of Medicine, Inc. 7‐Eleven, Inc. Alerus Financial Corporation A. O. Smith Corporation Alfa Laval, Inc. A. O. Water Treatment Alfa Mutual Insurance Company A123 Systems LLC All Nippon Airways Co., Ltd. AAA Club Alliance Inc. Allegis Group AAA National Office Alliance Data Systems ‐ Card Services AAA Northern California, Nevada and Utah Alliant Energy Corporation Aaron's Inc. Allianz Asset Management of America L.P. Abbott Laboratories ‐ Nutrition Allied Motion Technologies, Inc. Abloy Security, Inc. Allina Health System ABRA Auto Body & Glass Allina Health System ‐ New Ulm Medical Center Abt Associates, Inc. Allnex Group Accenture, Inc. Allnex USA Inc. Accolade Ally Financial, Inc. Accolade Wines North America, Inc. Alorica AccorHotels NA Alto‐Shaam ACH Food Companies, Inc. Altria Group, Inc. ACUITY Alyeska Pipeline Service Company Acushnet Holdings Corporation Alys Beach Adidas America, Inc. Amazing Grass Adient US LLC Amazon.com ADT, LLC Amcor Rigid Plastics Advance Auto Parts, Inc. Amedisys, Inc. Advance Local AMERICA CENTRAL CORPORATION Advanced Drainage Systems, Inc. American Airlines Group, Inc. Adventist Chippewa Valley Hospital American Augers Adventist Health System American Bureau of Shipping Adventist Health System GA Inc. American Century Investments Advocate Health Care American Century Investments ‐ CA Aegis Therapies, LLC American Dental Partners, Inc. AET Inc., Ltd. ‐ AET Offshore Services, Inc. American Enterprise Group, Inc. AET, Inc., Ltd. American Family Insurance Aetna, Inc. American Financial Group, Inc. Affinion Group Holdings, Inc. American Financial Group, Inc. ‐ Great American Financial Aflac, Inc. Resources, Inc. Aflac, Inc. ‐ Communicorp, Inc. American Financial Group, Inc. ‐ Great American Insurance Agero, Inc. Group AgFirst Farm Credit Bank American Financial Group, Inc. -
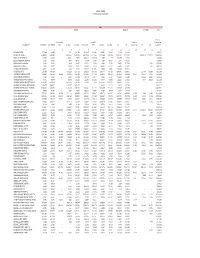
SFY 2021 Preliminary DSH Limit
Georgia Medicaid SFY 2021 DSH Limit Calculation Cost In‐State Uninsured Out‐of‐State Total Total Cost MCD Provider O/P FFS X‐ Uninsured Medicaid and Hospital Name I/P Medicaid O/P Medicaid Tax I/P MCO O/P MCO I/P FFS X‐Over Over I/P OME O/P OME I/P O/P Provider Tax I/P O/P Uninsured 23 4 5 6 7 8 9 10 11 12 13 14 15 16 17 APPLING HOSPITAL 1,225,498 429,453 ‐ 77,821 1,117,584 3,014,125 1,037,641 303,881 525,606 272,154 1,231,238 ‐ ‐ ‐ 9,235,001 AU MEDICAL CENTER 44,635,810 19,433,407 ‐ 36,159,992 24,189,104 34,672,223 20,107,546 5,729,685 2,958,814 36,181,697 22,021,951 ‐ 36,083,315 17,193,972 299,367,516 BACON COUNTY HOSPITAL 919,550 619,906 ‐ 724,901 763,062 1,481,300 1,813,704 6,526 998 517,763 965,078 ‐ ‐ ‐ 7,812,788 BLECKLEY MEMORIAL HOSPITAL 21,352 327,973 ‐ 15,949 557,264 24,495 484,754 2,330 324,720 3,772 861,330 ‐ ‐ ‐ 2,623,939 BROOKS COUNTY HOSPITAL 95,281 439,715 ‐ 16,803 689,827 147,011 481,326 46,586 177,382 63,671 1,007,838 ‐ ‐ 17,346 3,182,786 BURKE MEDICAL CENTER 145,022 358,377 ‐ 30,083 628,264 257,327 424,754 195,055 652,704 165,324 967,325 ‐ ‐ 3,741 3,827,976 CANDLER COUNTY HOSPITAL 359,077 518,789 ‐ 10,562 785,701 342,742 1,174,635 13,535 536,965 198,758 1,024,076 ‐ ‐ ‐ 4,964,840 CANDLER HOSPITAL 5,597,033 4,761,262 ‐ 9,321,814 8,547,941 6,535,284 4,802,788 7,895,000 6,780,238 6,387,992 9,836,675 ‐ 167,733 511,430 71,145,190 CARTERSVILLE MEDICAL CENTER 6,582,987 1,930,174 731,169 4,665,769 4,297,828 5,571,194 2,007,564 3,358,172 1,816,972 5,051,616 6,371,989 376,947 138,997 62,090 42,963,468 CHATUGE REGIONAL HOSPITAL -
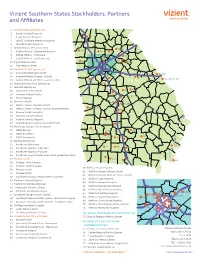
2018 Vizient Southern States Membership Map-Apr
Vizient Southern States Stockholders, Partners and Affiliates 1 Archbold Medical Center, Inc. 2 Brooks County Hospital 18 3 Grady General Hospital 19 16 4 John D. Archbold Memorial Hospital 5 Mitchell County Hospital 6 DeKalb Regional Health System 27, 28 28, 31 10 7 DeKalb Medical - Downtown Decatur 3433 35 30 8 DeKalb Medical - Hillandale 14, 63 33 30 9 DeKalb Medical - North Decatur 11 29 7 10 Floyd Medical Center 66 43 11 Polk Medical Center 12 Gwinnett Health System, Inc. 51, 52, 53 13 Glancy Rehabilitation Center 54, 56 69 14 Gwinnett Medical Center - Duluth 58 15 Gwinnett Medical Center - Lawrenceville 53, 55, 57, 59 70 GEORGIA 16 Hamilton Health Care System, Inc. 68 17 Houston Healthcare 25, 26 25 18 Houston Heart Institute 26 19 Houston Medical Center 24 24, 64 21, 22, 27 20 Perry Hospital 21 Navicent Health 23 17, 18, 19 22 Medical Center, Navicent Health 20, 21, 22 44, 45, 46 23 Medical Center of Peach County, Navicent Health 20 69 24 Monroe County Hospital 73 8, 9, 11, 61 25 Navicent Health Baldwin 23 26 Putnam General Hospital 40 68, 70 10 27 Rehabilitation Hospital, Navicent Health 75 28 Northeast Georgia Health System 29 NGMC Barrow 42 39 62 76 30 NGMC Braselton 66 31 NGMC Gainesville 37, 38 41 50, 52 6774 32 Northside Hospital 36 33 Northside Alpharetta 65 5 72 47, 48 34 Northside Hospital - Cherokee 51 41 34 35 50 35 Northside Hospital - Forsyth 40, 42, 43, 44 36 Northside Hospital Outpatient Center @ Meridian Mark 3 37 Phoebe Health 1, 4 3 260 49 38 Phoebe – Main Campus 6 48 46 39 Phoebe – North Campus 60 WellStar Health -

June 18, 2019 Requests for Extended Implementation/Performance Period
Use the links below for easy navigation Letters of Intent Letters of Intent - Expired New CON Applications Withdrawn CON Applications Disqualified CON Applications Office of Health Planning Pending/Complete Applications Pending Review/Incomplete CON Applications Recently Approved CON Applications Recently Denied CON Applications Appealed CON Projects Letters of Determination Requests for Miscellaneous Letters of Determination Appealed Determinations DET Review LNR Conversion CERTIFICATE OF NEED Requests for LNR for Diagnostic or Therapeutic Equipment Requests for LNR for Establishment of Physician-Owned Ambulatory Surgery Facilities Appealed LNRs June 12, 2019 – June 18, 2019 Requests for Extended Implementation/Performance Period Batching Notifications - Spring Need Projection Analyses New Batching Review Georgia Department of Community Health Winter Cycle Office of Health Planning Spring Cycle 2 Peachtree Street 5th Floor Non-Filed or Incomplete Surveys Atlanta, Georgia 30303-3159 Indigent-Charity Shortfalls (404) 656-0409 CON Filing Requirements (404) 656-0442 Fax (effective July 18, 2017) www.dch.georgia.gov Contact Information Verification of Lawful Presence within U.S. Periodic Reporting Requirements CON Thresholds Open Record Request Form Web Links www.GaMap2Care.info Certificate of Need Appeal Panel Letters of Intent LOI2019032 Hamilton Medical Center, Inc. dba Hamilton Medical Center Renovation/Expansion of Hospital Surgical Suite and Other Functions Received: 5/24/2019 Application must be submitted on: 6/24/2019 Site: 1200 Memorial -

Who Is My Provider Relations Representative?
Georgia Who is my Provider Relations Representative? Contact Information Territory/Facility/Group Andre Greenwood VP Provider Performance Programs [email protected] Statewide Provider Performance Programs Cell: 1-770-624-1373 Office: 1-770-913-2146 Contact Information Territory/Facility/Group Dawn Pierce Sr Mgr., Network Management [email protected] Statewide Physician Servicing for East, North and Atlanta Market Cell: 1-404-630-4357 Office: 1-404-630-4357 Contact Information Territory/Facility/Group Brian Bauereiss Manager, Network Development Statewide Value Based Partnerships [email protected] Cell: 1-478-796-4346 Contact Information Territory/Facility/Group Appointment Agendas BCG Medical Group (GA89), HCA Physicians (GA724), Amy Saltsman Kids First Pediatrics (Q#F), Pediatric Partners of Augusta (4BG), Network Performance Advisor Physicians Practice Group (VOP), Submit Appointment Agendas [email protected] Primary Care Association (U#9), Ramon Ramos (9RR), (Fax 1-813-675-2877) or email to: Cell: 1-912-224-4540 St. Joseph Candler Medical Group (YYH), [email protected] Office: 1-912-790-5622 South Georgia Physician Association (ABO) & University Health Link (2UN). Contact Information Territory/Facility/Group Appointment Agendas Patsy Chastain CAAP (JBO), Jeffeory H White (LWP), Peds Care PC (2TN), The Childrens Care Network (KF1C), Network Performance Advisor Submit Appointment Agendas The Childrens Care Network (4CHC), (Fax 1-813-675-2937) or email to: [email protected] The -

Healthcare Coalitions
Healthcare Coalitions Region Hospital A Hamilton Medical Center B Northeast Georgia Medical Center Dade Catoosa Fannin Towns C Floyd Medical Center Murray Union Rabun D Grady Health System Whitfield NORTHEAST E Piedmont Athens Regional Medical Center Walker HAMILTON A MEDICAL GEORGIA B 8 F Navicent Health Medical Center Gilmer Habersham CENTER MEDICAL CENTER ' G Augusta University Medical Center White Chattooga Lumpkin Stephens CH Fairview Park Hospital Gordon Pickens I Piedmont Columbus Regional Dawson Hall Hart J Memorial Health Univ. Medical Center Banks Franklin E FLOYD MEDICAL K Phoebe Putney Memorial Hospital CENTER Cherokee I Floyd Bartow Forsyth L WELLSTAR 0 C Jackson Madison M Memorial Satilla Health KENNESTONE Elbert 6 N Wellstar Kennestone Hospital HOSPITAL PIEDMONT ATHENS REGIONAL Polk D F"P1ed Children’s Healthcare of Atlanta Cobb Barrow MEDICAL CENTER Gwinnett Clarke DeKalb Oglethorpe COMMUNITY HOSPITAL Paulding Oconee Haralson N E Wilkes GRADY HEALTH Walton Lincoln REGIONAL COORDINATING HOSPITAL Douglas Fulton SYSTEM Rockdale Greene Carroll Clayton McDuffie Columbia Newton Taliaferro Morgan AUGUSTA UNIVERSITY Fayette Henry MEDICAL CENTER Warren Coweta Jasper Richmond Heard Putnam Spalding Butts Hancock Glascock G Pike Troup Lamar Jefferson Burke Meriwether Monroe Jones Baldwin Upson F Washington NAVICENT Wilkinson Jenkins HEALTH Bibb Harris Screven Talbot Crawford MEDICAL Johnson Twiggs CENTER FAIRVIEW PARK H Emanuel I Taylor HOSPITAL Muscogee Peach Laurens Piedmont Columbus Regional Bleckley Effingham Houston Treutlen -
Georgia Committee for Trauma Excellence
Approved November 8, 2018 Georgia Committee for Trauma Excellence MEETING MINUTES Thursday, August 16, 2018 King & Prince Resort Retreat Room 201 Arnold Rd St. Simons Island, Georgia MEMBERS PRESENT REPRESENTING Karen Hill, Vice Chair CHOA Egleston Gina Solomon, Past Chair Gwinnett Medical Center Regina Medeiros, GTC Chair & Special Projects Georgia Trauma Commission Erin Moorcones, Education (conference line) Grady Health System Kristal Smith, Injury Prevention Navicent Health Medical Center Anastasia Hartigan, PI Doctors Hospital of Augusta Tracy Johns, Registry Navicent Health Medical Center Kate Bailey, Pediatrics Memorial Hospital Sabrina Westbrook, Emergency Preparedness Piedmont Walton (conference line) OTHERS SIGNING IN REPRESENTING Amanda Wright Augusta University Patricia Newsome Augusta University Kyndra Holm Augusta University Mary Alice Aubrey (Via Conference Line) CHOA Egleston Karen Johnson CHOA Egleston Kellie Rowker CHOA Egleston Jon Johnson (Via Conference Line) CHOA Egleston Georgia Committee of Trauma Excellence Meeting Minutes: 16 August 2018 Page 1 Tai Woods (Via Conference Line) CHOA Egleston Nancy Friedel (Via Conference Line) CHOA Egleston Tracie Walton CHOA at Scottish Rite Jennifer Hutchinson (Via Conference Line) CHOA at Scottish Rite Tawnie Campbell (Via Conference Line) Coliseum Medical Center, HC Joni Napier Crisp Regional Hospital Janann Dunnavant Crisp Regional Hospital Tinyhra Harris (Via Conference Line) Doctor’s Hospital Kim Moore (Via Conference Line) Doctor’s Hospital Lisa Dukes (Via Conference Line) -
Patient Centered Data Analysis Uncovers State Hospital Rankings
Patient Centered Data analysis uncovers state hospital rankings ver the past several years healthcare reforms, Trend groups hospitals into Teaching Hospitals, whose primary particularly around unsustainable spending, mission is teaching regardless of size and are certified by the the uninsured population and quality out- Association of American Medical Colleges Council of Teaching comes, have been widely discussed and de- Hospitals and Health Systems; Large Hospitals (250+ patient O bated. While this talk is ongoing, hospitals beds); Medium Hospitals (100 to 249 beds); and Small Hospitals continue to operate in a complex regulatory (less than 100 beds). The American Hospital Directory provided environment with uncertain policy outcomes, the hospital bed size. all while making investments to enhance effi- The rankings are based on CMS data downloaded Aug. 3, ciency and deliver quality patient care. 2019. Analysis of the data was completed for Georgia Trend For this list, which includes hospitals that provide a range by independent consultant Mark A. Thompson, professor of services, Georgia Trend evaluated each hospital in the state and associate dean of the Hull College of Business at Au- that participates in the Centers for Medicare and Medicaid Ser- gusta University. vices (CMS) Hospital Value Based Purchasing program. The While these hospital rankings on performance are useful CMS program does not include VA medical centers, children’s and provide valuable information, there are many factors hospitals, critical access hospitals and long-term care facilities. that consumers should consider when deciding where to go A total performance score based on information including for their healthcare needs. Always consult your healthcare clinical process, patient experience, outcome and efficiency provider about your and your family’s healthcare needs. -

2017 Legislative Session Was a Good One for Our Hospitals
Letter from the President/CEO Monty Veazey Board of Directors Corporate Sponsors Large Hospital of the Year Small Hospital of the Year Annual Conference Highlights General P.O. Box 1572, Tifton, Georgia 31793 Phone (229) 386-8660 Fax (229) 386-8662 Our job as an Alliance is to make sure our leaders understand the very real challenges we all face and to remind them that the decisions they make with regard to healthcare policy have very real consequences. Thanks to your support, active engagement and the heroic work you do in the communities you serve, we continue to achieve that goal. The 2017 legislative session was a good one for our hospitals. One of the very first actions taken by legislative leaders was the renewal of the provider fee and they also made changes to the rural hospital tax credit to encourage greater participation. We faced what has become an annual assault on Georgia’s Certificate of Need, laws were once again successful in our efforts to stop that legislation. Since the legislature adjourned, we have presented to special committees of both the House and Senate about the many challenges facing rural hospitals and talked with them about the distance to care, the cost of insurance or lack of coverage altogether and the horrifying opioid epidemic are impacting the lives of Georgians throughout our state.And we have made it very clear that now is not the time to make changes to Certificate of Need laws, take away existing tax exemptions, or end the disproportionate share program. I wish I could tell you that there is a clear path ahead for any of the issues mentioned - or even that these are the only challenges we will continue to face, but that wouldn’t be true.