Meta-Transcriptomic Discovery of a Divergent Circovirus and a Chaphamaparvovirus in Captive Reptiles with Proliferative Respiratory Syndrome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genome Sequencing by Random Priming Methods for Viral Identification
Genome sequencing by random priming methods for viral identification Rosseel Toon Dissertation submitted in fulfillment of the requirements for the degree of Doctor of Philosophy (PhD) in Veterinary Sciences, Faculty of Veterinary Medicine, Ghent University, 2015 Promotors: Dr. Steven Van Borm Prof. Dr. Hans Nauwynck “The real voyage of discovery consist not in seeking new landscapes, but in having new eyes” Marcel Proust, French writer, 1923 Table of contents Table of contents ....................................................................................................................... 1 List of abbreviations ................................................................................................................. 3 Chapter 1 General introduction ................................................................................................ 5 1. Viral diagnostics and genomics ....................................................................................... 7 2. The DNA sequencing revolution ................................................................................... 12 2.1. Classical Sanger sequencing .................................................................................. 12 2.2. Next-generation sequencing ................................................................................... 16 3. The viral metagenomic workflow ................................................................................. 24 3.1. Sample preparation ............................................................................................... -

Trimeresurus Sp
Trimeresurus sp. Copyright: Auszug aus Datenbank der Toxikologischen Abteilung der II. Medizinischen Klinik München; Toxinfo von Kleber JJ , Ganzert M, Zilker Th; Ausgabe 2002; erstellt Wagner Ph, Kleber JJ; Korthals Altes 1999 TOXIKOLOGIE: bei allen Arten von Trimeresurus Sp kommt es immer zu Lokalsymptomen bis Nekrose; zu rechnen ist mit außerdem mit Gerinnungsstörungen, Schocksymptomen T. ALBOLABRIS: massive Lokalsymptome, Gerinnungsstörung leicht bei 30%, stark bei 10% (16); Letalität in Thailand 3% (12) T. FLAVOVIRIDIS: vor Antiserum-Zeit 15% Letalität (12) starke Schwellung + Nekrosen, Schock, keine Gerinnungsstörungen bisher berichtet (11,12) T. GRAMINEUS: Schwellung; keine Nekrosen, keine Gerinnungsstörungen berichtet (1,14) T. KANBURIENSIS: Schwellung, Schock, Gerinnungsstörung (12) T. MUCROSQUAMATUS: Schwellung, Gerinnungsstörungen (12) T. POPEIORUM: Lokalsymptome; sehr geringe Gerinnungsstörung mit normalem Fibrinogen + Thrombo (15) T. PURPUREOMACULATUS: Schwellung, Nekrose, Gerinnungsstörung bis 40% (12,15) T. WAGLERI: Schwellung, Gerinnungsstörung (15) SYMPTOME: erste Vergiftungssymptome direkt nach dem Biß (sofortiger Schmerz, Schwellung entwickelt sich in den ersten 2-4 h) (2); meist starke Schwellung (häufig halbes bis ganzes Glied), bis ca. eine Woche anhaltend; Lymphangitis und schmerzhafte Lymphknotenschwellung (1,2,5,6) ; lokale subkutane Hämorrhagie, gelegentlich Blasenbildung und Hautnekrosen (1,2); bei T. flavoviridis auch Muskelnekrosen und Kompartmentsyndrom (3,12) MUND: lokal nach Giftaussaugen Schwellung an Lippe + Zunge bei T. albolabris (12) COR: selten Butdruckabfall, Schock (11,12); selten EKG-Veränderungen bei T. mucrosquamatus (12) LABOR: Thrombin ähnliche Aktivität führt zur Defibrinierung bis Verbrauchskoagulopathie mit Hypo- bis Afibrinogenämie, Thrombopenie (auch erst nach 12h Latenz) (1,2,4,13,16); Aktivierung der Fibrinolyse mit später Plasminerniedrigung (16); Leukozytose SONST: häufig Übelkeit, Erbrechen, Bauchschmerzen (11, 12); selten Nierenschädigung berichtet bei T. -

On Trimeresurus Sumatranus
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/266262458 On Trimeresurus sumatranus (Raffles, 1822), with the designation of a neotype and the description of a new species of pitviper from Sumatra (Squamata: Viperidae: Crotalinae) Article in Amphibian and Reptile Conservation · September 2014 CITATIONS READS 4 360 3 authors, including: Gernot Vogel Irvan Sidik Independent Researcher Indonesian Institute of Sciences 102 PUBLICATIONS 1,139 CITATIONS 12 PUBLICATIONS 15 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Save Vietnam Biodiversity View project Systematics of the genus Pareas View project All content following this page was uploaded by Gernot Vogel on 01 October 2014. The user has requested enhancement of the downloaded file. Comparative dorsal view of the head of Trimeresurus gunaleni spec. nov. (left) and T. sumatranus (right). Left from above: male, female (holotype), male, all alive, from Sumatra Utara Province, Sumatra. Right: adult female alive from Bengkulu Province, Su- matra, adult male alive from Bengkulu Province, Sumatra, preserved female from Borneo. Photos: N. Maury. Amphib. Reptile Conserv. | amphibian-reptile-conservation.org (1) September 2014 | Volume 8 | Number 2 | e80 Copyright: © 2014 Vogel et al. This is an open-access article distributed under the terms of the Creative Commons Attribution–NonCommercial–NoDerivs 3.0 Unported License, Amphibian & Reptile Conservation which permits -

The Intestinal Virome of Malabsorption Syndrome-Affected and Unaffected
Virus Research 261 (2019) 9–20 Contents lists available at ScienceDirect Virus Research journal homepage: www.elsevier.com/locate/virusres The intestinal virome of malabsorption syndrome-affected and unaffected broilers through shotgun metagenomics T ⁎ Diane A. Limaa, , Samuel P. Cibulskib, Caroline Tochettoa, Ana Paula M. Varelaa, Fabrine Finklera, Thais F. Teixeiraa, Márcia R. Loikoa, Cristine Cervac, Dennis M. Junqueirad, Fabiana Q. Mayerc, Paulo M. Roehea a Laboratório de Virologia, Departamento de Microbiologia, Imunologia e Parasitologia, Instituto de Ciências Básicas da Saúde (ICBS), Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil b Laboratório de Virologia, Faculdade de Veterinária, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil c Laboratório de Biologia Molecular, Instituto de Pesquisas Veterinárias Desidério Finamor (IPVDF), Eldorado do Sul, RS, Brazil d Centro Universitário Ritter dos Reis - UniRitter, Health Science Department, Porto Alegre, RS, Brazil ARTICLE INFO ABSTRACT Keywords: Malabsorption syndrome (MAS) is an economically important disease of young, commercially reared broilers, Enteric disorders characterized by growth retardation, defective feather development and diarrheic faeces. Several viruses have Virome been tentatively associated to such syndrome. Here, in order to examine potential associations between enteric Broiler chickens viruses and MAS, the faecal viromes of 70 stool samples collected from diseased (n = 35) and healthy (n = 35) High-throughput sequencing chickens from seven flocks were characterized and compared. Following high-throughput sequencing, a total of 8,347,319 paired end reads, with an average of 231 nt, were generated. Through analysis of de novo assembled contigs, 144 contigs > 1000 nt were identified with hits to eukaryotic viral sequences, as determined by GenBank database. -

Alma Mater Studiorum – Università Di Bologna
Alma Mater Studiorum – Università di Bologna DOTTORATO DI RICERCA IN Scienze Biotecnologiche e Farmaceutiche Ciclo XXXI Settore Concorsuale: 06/A3 - MICROBIOLOGIA E MICROBIOLOGIA CLINICA Settore Scientifico Disciplinare: MED/07 - MICROBIOLOGIA E MICROBIOLOGIA CLINICA TITOLO TESI “Human Parvovirus B19: from the development of a reverse genetics system to antiviral strategies” Presentata da: Ilaria Conti Matricola n. 0000772499 Coordinatore Dottorato Supervisore Chiar.mo Prof. Chiar.mo Prof. Santi Mario Spampinato Giorgio Gallinella Esame finale anno 2019 Abstract Dott. Ilaria Conti Supervisor: Prof. Giorgio Gallinella PhD course: Scienze Biotecnologiche e Farmaceutiche, XXXI ciclo Thesis: “Human Parvovirus B19: from the development of a reverse genetics system to antiviral strategies” Human Parvovirus B19 (B19V) is a human pathogenic virus which belongs to the Parvoviridae family. It is worldwide distributed and is responsible of various clinical manifestations in human, although neither an antiviral therapy nor a vaccine are available. The virus is not well adapted to grow in cellular cultures and this causes difficulties for its propagation, maintaining and characterization. B19V has a narrow tropism for the erythroid progenitor cells of human bone marrow and very few cellular systems can support the viral replication (such as, UT7/EpoS1 cells which is a megakaryoblastoid cell line). In this research, a reverse genetic approach was developed to allow the generation of mature and infectious viral particles from an established consensus sequence. Synthetic clones that differ for the length and isomerism of their terminal regions (ITRs) were constructed. After their transfection in UT7/EpoS1 cells, the obtained viral particles were used to infect EPCs (erythroid progenitor cells) in serial passages, in order to evaluate the capability for each clone to generate a new viral stock and to propagate it. -

Two Novel Bocaparvovirus Species Identified in Wild Himalayan
SCIENCE CHINA Life Sciences SPECIAL TOPIC: Emerging and re-emerging viruses ............................. December 2017 Vol.60 No. 12: 1348–1356 •RESEARCH PAPER• ...................................... https://doi.org/10.1007/s11427-017-9231-4 Two novel bocaparvovirus species identified in wild Himalayan marmots Yuanyun Ao1†, Xiaoyue Li2†, Lili Li1, Xiaolu Xie3, Dong Jin4, Jiemei Yu1*, Shan Lu4* & Zhaojun Duan1* 1National Institute for Viral Diseases Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing 100052, China; 2Laboratory Department, the First People’s Hospital of Anqing, Anqing 246000, China; 3Peking Union Medical College Hospital, Beijing 100730, China; 4National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing 102206, China Received September 10, 2017; accepted September 16, 2017; published online December 1, 2017 Bocaparvovirus (BOV) is a genetically diverse group of DNA viruses and a possible cause of respiratory, enteric, and neuro- logical diseases in humans and animals. Here, two highly divergent BOVs (tentatively named as Himalayan marmot BOV, HMBOV1 and HMBOV2) were identified in the livers and feces of wild Himalayan marmots in China, by viral metagenomic analysis. Five of 300 liver samples from Himalayan marmots were positive for HMBOV1 and five of 99 fecal samples from these animals for HMBOV2. Their nearly complete genome sequences are 4,672 and 4,887 nucleotides long, respectively, with a standard genomic organization and containing protein-coding motifs typical for BOVs. Based on their NS1, NP1, and VP1, HMBOV1 and HMBOV2 are most closely related to porcine BOV SX/1-2 (approximately 77.0%/50.0%, 50.0%/53.0%, and 79.0%/54.0% amino acid identity, respectively). -
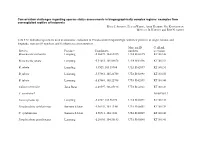
Conservation Challenges Regarding Species Status Assessments in Biogeographically Complex Regions: Examples from Overexploited Reptiles of Indonesia KYLE J
Conservation challenges regarding species status assessments in biogeographically complex regions: examples from overexploited reptiles of Indonesia KYLE J. SHANEY, ELIJAH WOSTL, AMIR HAMIDY, NIA KURNIAWAN MICHAEL B. HARVEY and ERIC N. SMITH TABLE S1 Individual specimens used in taxonomic evaluation of Pseudocalotes tympanistriga, with their province of origin, latitude and longitude, museum ID numbers, and GenBank accession numbers. Museum ID GenBank Species Province Coordinates numbers accession Bronchocela cristatella Lampung -5.36079, 104.63215 UTA R 62895 KT180148 Bronchocela jubata Lampung -5.54653, 105.04678 UTA R 62896 KT180152 B. jubata Lampung -5.5525, 105.18384 UTA R 62897 KT180151 B. jubata Lampung -5.57861, 105.22708 UTA R 62898 KT180150 B. jubata Lampung -5.57861, 105.22708 UTA R 62899 KT180146 Calotes versicolor Jawa Barat -6.49597, 106.85198 UTA R 62861 KT180149 C. versicolor* NC009683.1 Gonocephalus sp. Lampung -5.2787, 104.56198 UTA R 60571 KT180144 Pseudocalotes cybelidermus Sumatra Selatan -4.90149, 104.13401 UTA R 60551 KT180139 P. cybelidermus Sumatra Selatan -4.90711, 104.1348 UTA R 60549 KT180140 Pseudocalotes guttalineatus Lampung -5.28105, 104.56183 UTA R 60540 KT180141 P. guttalineatus Sumatra Selatan -4.90681, 104.13457 UTA R 60501 KT180142 Pseudocalotes rhammanotus Lampung -4.9394, 103.85292 MZB 10804 KT180147 Pseudocalotes species 4 Sumatra Barat -2.04294, 101.31129 MZB 13295 KT211019 Pseudocalotes tympanistriga Jawa Barat -6.74181, 107.0061 UTA R 60544 KT180143 P. tympanistriga Jawa Barat -6.74181, 107.0061 UTA R 60547 KT180145 Pogona vitticeps* AB166795.1 *Entry to GenBank by previous authors TABLE S2 Reptile species currently believed to occur Java and Sumatra, Indonesia, with IUCN Red List status, and certainty of occurrence. -

A Metagenomic Comparison of Endemic Viruses from Broiler Chickens with Runting Stunting Syndrome and from Normal Birds
A metagenomic comparison of endemic viruses from broiler chickens with runting stunting syndrome and from normal birds Devaney, R., Trudgett, J., Trudgett, A., Meharg, C., & Smyth, V. (2016). A metagenomic comparison of endemic viruses from broiler chickens with runting stunting syndrome and from normal birds. Avian Pathology. https://doi.org/10.1080/03079457.2016.1193123 Published in: Avian Pathology Document Version: Peer reviewed version Queen's University Belfast - Research Portal: Link to publication record in Queen's University Belfast Research Portal Publisher rights © 2016, Taylor and Francis This is an Accepted Manuscript of an article published by Taylor & Francis in Avian Pathology on 23rd May 2016, available online: http://www.tandfonline.com/doi/abs/10.1080/03079457.2016.1193123 General rights Copyright for the publications made accessible via the Queen's University Belfast Research Portal is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The Research Portal is Queen's institutional repository that provides access to Queen's research output. Every effort has been made to ensure that content in the Research Portal does not infringe any person's rights, or applicable UK laws. If you discover content in the Research Portal that you believe breaches copyright or violates any law, please contact [email protected]. Download date:24. Sep. 2021 Avian Pathology ISSN: -

Parvoviridae Hanafy .M.Madbouly Professor of Virology Faculty of Veterinary Medicine Beni-Suef University
Parvoviridae Hanafy .M.Madbouly Professor of Virology Faculty of Veterinary Medicine Beni-Suef University Definitions of the virus causative agents of several animal diseases. are most severe in fetuses and neonates. Parvovirus infections of the fetus or newborn during organogenesis may result in developmental defects. Replication of parvovirus is restricted in hemopoietic precursors, lymphocytes, and progenitor cells of intestinal mucosa of older animals. 2/38 Definitions of the virus cause acute infections for a few days, others persist for long periods in the feces of apparently robust host immune responses. Human Parvovirus B19 replication occurs only in human erythrocyte precursors The virus is found to survive in feces and other organic material such as soil for over 10 years. It survives extremely low and high temperatures. 3/38 Physical Properties of the virus . Family: Parvoviridae with two subfamilies . Parvovirinae. (vertebrate ). has 5 genera . Densovirinae. (invertebrate). Has3 genera .non-enveloped, 25 nm in diameter .Capsid: icosahederal. .Genome ssDNA Diseases caused by parvoviruses • As of 2014, there were no known human viruses in the remaining three recognized genera: • vi) Amdoparvovirus (e.g. Aleutian mink disease virus), • vii) Aveparvovirus (e.g. chicken parvovirus) and • viii) Copiparvovirus (e.g. bovine parvovirus 2). • Mouse parvovirus 1, however, causes no symptoms but can contaminate immunology experiments in biological research laboratories. • Porcine parvovirus causes a reproductive disease in swine known as SMEDI, which stands for stillbirth, mummification, embryonic death, and infertility. Diseases caused by parvoviruses • Many mammalian species sustain infection by multiple parvoviruses. • Feline panleukopenia is common in kittens and causes fever, low white blood cell count, diarrhea, and death. -

Molecular Diagnostic of Chicken Parvovirus (Chpv) Affecting Broiler Flocks in Ecuador
Brazilian Journal of Poultry Science Revista Brasileira de Ciência Avícola ISSN 1516-635X Oct - Dec 2018 / v.20 / n.4 / 643-650 Molecular Diagnostic of Chicken Parvovirus (ChPV) Affecting Broiler Flocks in Ecuador http://dx.doi.org/10.1590/1806-9061-2018-0730 Author(s) ABSTRACT I,II De la Torre D https://orcid.org/0000-0002-8306-9200 Enteric diseases affect poultry and cause important economic Nuñez LFNI,II losses in many countries worldwide. Avian parvovirus has been linked Puga BII https://orcid.org/0000-0002-4444-0054 I Parra SHS https://orcid.org/0000-0002-8609-4399 to enteric conditions, such as malabsorption and runting-stunting Astolfi-Ferreira CSI syndrome (RSS), characterized by diarrhoea, and reduced weight gain https://orcid.org/0000-0001-5032-2735 I and growth retardation. In 2013 and 2016, 79 samples were collected Ferreira AJP https://orcid.org/0000-0002-0086-5181 from different organs of chickens in Ecuador that exhibited signs of I Department of Pathology, School of Veterinary Medicine, University of São Paulo, Av. Prof. diarrhea and stunting syndrome, and analysed for the presence of Orlando Marques de Paiva, 87, 05588-000, São chicken parvovirus (ChPV). The detection method of ChPV applied was Paulo, Brazil, and II School of Veterinary Medicine and Animal Polymerase Chain Reaction (PCR), using primers designed from the Science, Central University of Ecuador, Quito, conserved region of the viral genome that encodes the non-structural Ecuador protein NS1. Out of the 79 samples, 50.6% (40/79) were positive for ChPV, and their nucleotide and amino acid sequences were analysed to determine their phylogenetic relationship with the sequences reported in the United States, Canada, China, South Korea, Croatia, Poland, Hungary, and Brazil. -

Evidence to Support Safe Return to Clinical Practice by Oral Health Professionals in Canada During the COVID-19 Pandemic: a Repo
Evidence to support safe return to clinical practice by oral health professionals in Canada during the COVID-19 pandemic: A report prepared for the Office of the Chief Dental Officer of Canada. November 2020 update This evidence synthesis was prepared for the Office of the Chief Dental Officer, based on a comprehensive review under contract by the following: Paul Allison, Faculty of Dentistry, McGill University Raphael Freitas de Souza, Faculty of Dentistry, McGill University Lilian Aboud, Faculty of Dentistry, McGill University Martin Morris, Library, McGill University November 30th, 2020 1 Contents Page Introduction 3 Project goal and specific objectives 3 Methods used to identify and include relevant literature 4 Report structure 5 Summary of update report 5 Report results a) Which patients are at greater risk of the consequences of COVID-19 and so 7 consideration should be given to delaying elective in-person oral health care? b) What are the signs and symptoms of COVID-19 that oral health professionals 9 should screen for prior to providing in-person health care? c) What evidence exists to support patient scheduling, waiting and other non- treatment management measures for in-person oral health care? 10 d) What evidence exists to support the use of various forms of personal protective equipment (PPE) while providing in-person oral health care? 13 e) What evidence exists to support the decontamination and re-use of PPE? 15 f) What evidence exists concerning the provision of aerosol-generating 16 procedures (AGP) as part of in-person -

A Phylogeny and Revised Classification of Squamata, Including 4161 Species of Lizards and Snakes
BMC Evolutionary Biology This Provisional PDF corresponds to the article as it appeared upon acceptance. Fully formatted PDF and full text (HTML) versions will be made available soon. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes BMC Evolutionary Biology 2013, 13:93 doi:10.1186/1471-2148-13-93 Robert Alexander Pyron ([email protected]) Frank T Burbrink ([email protected]) John J Wiens ([email protected]) ISSN 1471-2148 Article type Research article Submission date 30 January 2013 Acceptance date 19 March 2013 Publication date 29 April 2013 Article URL http://www.biomedcentral.com/1471-2148/13/93 Like all articles in BMC journals, this peer-reviewed article can be downloaded, printed and distributed freely for any purposes (see copyright notice below). Articles in BMC journals are listed in PubMed and archived at PubMed Central. For information about publishing your research in BMC journals or any BioMed Central journal, go to http://www.biomedcentral.com/info/authors/ © 2013 Pyron et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes Robert Alexander Pyron 1* * Corresponding author Email: [email protected] Frank T Burbrink 2,3 Email: [email protected] John J Wiens 4 Email: [email protected] 1 Department of Biological Sciences, The George Washington University, 2023 G St.