And Blue and Gold Macaws (Ara Ararauna)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

§4-71-6.5 LIST of CONDITIONALLY APPROVED ANIMALS November
§4-71-6.5 LIST OF CONDITIONALLY APPROVED ANIMALS November 28, 2006 SCIENTIFIC NAME COMMON NAME INVERTEBRATES PHYLUM Annelida CLASS Oligochaeta ORDER Plesiopora FAMILY Tubificidae Tubifex (all species in genus) worm, tubifex PHYLUM Arthropoda CLASS Crustacea ORDER Anostraca FAMILY Artemiidae Artemia (all species in genus) shrimp, brine ORDER Cladocera FAMILY Daphnidae Daphnia (all species in genus) flea, water ORDER Decapoda FAMILY Atelecyclidae Erimacrus isenbeckii crab, horsehair FAMILY Cancridae Cancer antennarius crab, California rock Cancer anthonyi crab, yellowstone Cancer borealis crab, Jonah Cancer magister crab, dungeness Cancer productus crab, rock (red) FAMILY Geryonidae Geryon affinis crab, golden FAMILY Lithodidae Paralithodes camtschatica crab, Alaskan king FAMILY Majidae Chionocetes bairdi crab, snow Chionocetes opilio crab, snow 1 CONDITIONAL ANIMAL LIST §4-71-6.5 SCIENTIFIC NAME COMMON NAME Chionocetes tanneri crab, snow FAMILY Nephropidae Homarus (all species in genus) lobster, true FAMILY Palaemonidae Macrobrachium lar shrimp, freshwater Macrobrachium rosenbergi prawn, giant long-legged FAMILY Palinuridae Jasus (all species in genus) crayfish, saltwater; lobster Panulirus argus lobster, Atlantic spiny Panulirus longipes femoristriga crayfish, saltwater Panulirus pencillatus lobster, spiny FAMILY Portunidae Callinectes sapidus crab, blue Scylla serrata crab, Samoan; serrate, swimming FAMILY Raninidae Ranina ranina crab, spanner; red frog, Hawaiian CLASS Insecta ORDER Coleoptera FAMILY Tenebrionidae Tenebrio molitor mealworm, -

TAG Operational Structure
PARROT TAXON ADVISORY GROUP (TAG) Regional Collection Plan 5th Edition 2020-2025 Sustainability of Parrot Populations in AZA Facilities ...................................................................... 1 Mission/Objectives/Strategies......................................................................................................... 2 TAG Operational Structure .............................................................................................................. 3 Steering Committee .................................................................................................................... 3 TAG Advisors ............................................................................................................................... 4 SSP Coordinators ......................................................................................................................... 5 Hot Topics: TAG Recommendations ................................................................................................ 8 Parrots as Ambassador Animals .................................................................................................. 9 Interactive Aviaries Housing Psittaciformes .............................................................................. 10 Private Aviculture ...................................................................................................................... 13 Communication ........................................................................................................................ -

In Search of the Extinct Hutia in Cave Deposits of Isla De Mona, P.R. by Ángel M
In Search of the Extinct Hutia in Cave Deposits of Isla de Mona, P.R. by Ángel M. Nieves-Rivera, M.S. and Donald A. McFarlane, Ph.D. Isolobodon portoricensis, the extinct have been domesticated, and its abundant (14C) date was obtained on charcoal and Puerto Rican hutia (a large guinea-pig like remains in kitchen middens indicate that it bone fragments from Cueva Negra, rodent), was about the size of the surviving formed part of the diet for the early settlers associated with hutia bones (Frank, 1998). Hispaniolan hutia Plagiodontia (Rodentia: (Nowak, 1991; Flemming and MacPhee, This analysis yielded an uncorrected 14C age Capromydae). Isolobodon portoricensis was 1999). This species of hutia was extinct, of 380 ±60 before present, and a corrected originally reported from Cueva Ceiba (next apparently shortly after the coming of calendar age of 1525 AD, (1 sigma range to Utuado, P.R.) in 1916 by J. A. Allen (1916), European explorers, according to most 1480-1655 AD). This date coincides with the and it is known today only by skeletal remains historians. final occupation of Isla de Mona by the Taino from Hispaniola (Dominican Republic, Haiti, The first person to take an interest in the Indians (1578 AD; Wadsworth 1977). The Île de la Gonâve, ÎIe de la Tortue), Puerto faunal remains of the caves of Isla de Mona purpose of this article is to report some new Rico (mainland, Isla de Mona, Caja de was mammalogist Harold E. Anthony, who paleontological discoveries of the Puerto Muertos, Vieques), the Virgin Islands (St. in 1926 collected the first Puerto Rican hutia Rican hutia in cave deposits of Isla de Mona Croix, St. -
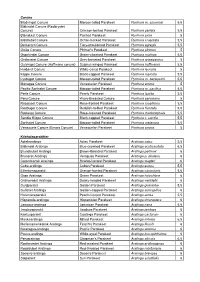
Rødbrystet Conure
Conure Blodvinget Conure Maroon -tailed Parakeet Pyrrhura m. souancei 5,5 Blåkindet Conure (Rødbrystet Conure) Crimson-bellied Parakeet Pyrrhura perlata 5,5 Blånakket Conure Painted Parakeet Pyrrhura picta 5 Blåstrubet Conure Ochre-marked Parakeet Pyrrhura cruentata 5,5 Demerara Conure Fiery-shouldered Parakeet Pyrrhura egregia 5,5 Goiás Conure Pfrimer's Parakeet Pyrrhura pfrimeri 5 Grønkindet Conure Green-cheeked Parakeet Pyrrhura molinae 5,5 Gråbrystet Conure Grey-breasted Parakeet Pyrrhura griseipectus 5 Gulvinget Conure (Hoffmans conure) Sulphur-winged Parakeet Pyrrhura hoffmanni 5,5 Hvidøret Conure White-eared Parakeet Pyrrhura leucotis 5 Klippe Conure Black-capped Parakeet Pyrrhura rupicola 5,5 Lysbuget Conure Maroon-tailed Parakeet Pyrrhura m. berlepschi 5,5 Monagas Conure Venezuelan Parakeet Pyrrhura emma 5 Pacific Sorthalet Conure Maroon-tailed Parakeet Pyrrhura m. pacifica 5,5 Perle Conure Pearly Parakeet Pyrrhura lepida 5,5 Peru Conure Wavy-Breasted Conure Pyrrhura peruviana 5 Rosaisset Conure Rose-fronted Parakeet Pyrrhura roseifrons 5,5 Rødbuget Conure Reddish-bellied Parakeet Pyrrhura frontalis 5,5 Rødisset Conure Rose-crowned Parakeet Pyrrhura rhodocephala 5,5 Sandia Klippe Conure Black-capped Parakeet Pyrrhura r. sandia 5,5 Sorthalet Conure Maroon-tailed Parakeet Pyrrhura melanura 5,5 Venezuela Conure (Emma Conure) Venezuelan Parakeet Pyrrhura emma 5 Kilehaleparakitter Aztekaratinga Aztec Parakeet Aratinga astec 5,5 Blåkindet Aratinga Blue-crowned Parakeet Aratinga acuticaudata 6,5 Brunstrubet Aratinga Brown-throated Parakeet -

Vogelliste Venezuela
Vogelliste Venezuela Datum: www.casa-vieja-merida.com (c) Beobachtungstage: 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Birdlist VENEZUELA copyrightBeobachtungsgebiete: Henri Pittier Azulita / Catatumbo La Altamira St Domingo Paramo Los Llanos Caura Sierra de Imataca Sierra de Lema + Gran Sabana Sucre Berge und Kueste Transfers Andere - gesehen gesehen an wieviel Tagen TINAMIFORMES: Tinamidae - Steißhühner 0 1 Tawny-breasted Tinamou Nothocercus julius Gelbbrusttinamu 0 2 Highland Tinamou Nothocercus bonapartei Bergtinamu 0 3 Gray Tinamou Tinamus tao Tao 0 4 Great Tinamou Tinamus major Großtinamu x 0 5 White-throated Tinamou Tinamus guttatus Weißkehltinamu 0 6 Cinereous Tinamou Crypturellus cinereus Grautinamu x x 0 7 Little Tinamou Crypturellus soui Brauntinamu x x x 0 8 Tepui Tinamou Crypturellus ptaritepui Tepuitinamu by 0 9 Brown Tinamou Crypturellus obsoletus Kastanientinamu 0 10 Undulated Tinamou Crypturellus undulatus Wellentinamu 0 11 Gray-legged Tinamou Crypturellus duidae Graufußtinamu 0 12 Red-legged Tinamou Crypturellus erythropus Rotfußtinamu birds-venezuela.dex x 0 13 Variegated Tinamou Crypturellus variegatus Rotbrusttinamu x x x 0 14 Barred Tinamou Crypturellus casiquiare Bindentinamu 0 ANSERIFORMES: Anatidae - Entenvögel 0 15 Horned Screamer Anhima cornuta Hornwehrvogel x 0 16 Northern Screamer Chauna chavaria Weißwangen-Wehrvogel x 0 17 White-faced Whistling-Duck Dendrocygna viduata Witwenpfeifgans x 0 18 Black-bellied Whistling-Duck Dendrocygna autumnalis Rotschnabel-Pfeifgans x 0 19 Fulvous Whistling-Duck Dendrocygna bicolor -

AWI-WL-Hyacinth-Macaw-Comments
January 27, 2017 VIA Electronic Submission to: http://www.regulations.gov Public Comments Processing Attn: FWS–R9– ES–2012–0013 Division of Policy and Directives Management U.S. Fish and Wildlife Service 4401 N. Fairfax Drive Arlington, VA 22203 Dear Branch Chief Van Norman: Thank you for the opportunity to comment on the proposed threatened listing and draft 4(d) rule for the hyacinth macaw (Anodorhynchus hyacinthinus). These comments are submitted on behalf of the Center for Biological Diversity (Center) and the Animal Welfare Institute (AWI). The Center is a nonprofit conservation organization with more than 1,200,000 members and online activists dedicated to the protection of endangered species and wild places. The Center and its members have a long standing interest in the conservation of foreign species and their habitat, including the hyacinth macaw. AWI is a nonprofit, charitable organization founded in 1951 and dedicated to reducing animal suffering caused by people. AWI has been engaged in efforts to confront issues associated with wildlife trade, particularly the commercial trade in wild-caught birds. We have had a long-standing interest in the conservation of the hyacinth macaw and concern for the detrimental effects of trade coupled with habitat loss on the species. We vehemently disagree with the suggestion that the hyacinth macaw should be listed as threatened instead of endangered under the Endangered Species Act (ESA or Act). Habitat loss is still a significant threat to this species, as is the pet trade. Moreover, the United States Fish and Wildlife Service’s (USFWS or Service) Significant Portion of the Range Policy (SPOR policy) is unlawful as evidenced by the agency’s decision here that the birds are threatened throughout their range and therefore a significant portion of their range need not be analyzed. -

Juan Cristóbal Gundlach's Collections of Puerto Rican Birds with Special
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Zoosystematics and Evolution Jahr/Year: 2015 Band/Volume: 91 Autor(en)/Author(s): Frahnert Sylke, Roman Rafela Aguilera, Eckhoff Pascal, Wiley James W. Artikel/Article: Juan Cristóbal Gundlach’s collections of Puerto Rican birds with special regard to types 177-189 Creative Commons Attribution 4.0 licence (CC-BY); original download https://pensoft.net/journals Zoosyst. Evol. 91 (2) 2015, 177–189 | DOI 10.3897/zse.91.5550 museum für naturkunde Juan Cristóbal Gundlach’s collections of Puerto Rican birds with special regard to types Sylke Frahnert1, Rafaela Aguilera Román2, Pascal Eckhoff1, James W. Wiley3 1 Museum für Naturkunde, Leibniz-Institut für Evolutions- und Biodiversitätsforschung, Invalidenstraße 43, D-10115 Berlin, Germany 2 Instituto de Ecología y Sistemática, La Habana, Cuba 3 PO Box 64, Marion Station, Maryland 21838-0064, USA http://zoobank.org/B4932E4E-5C52-427B-977F-83C42994BEB3 Corresponding author: Sylke Frahnert ([email protected]) Abstract Received 1 July 2015 The German naturalist Juan Cristóbal Gundlach (1810–1896) conducted, while a resident Accepted 3 August 2015 of Cuba, two expeditions to Puerto Rico in 1873 and 1875–6, where he explored the Published 3 September 2015 southwestern, western, and northeastern regions of this island. Gundlach made repre sentative collections of the island’s fauna, which formed the nucleus of the first natural Academic editor: history museums in Puerto Rico. When the natural history museums closed, only a few Peter Bartsch specimens were passed to other institutions, including foreign museums. None of Gund lach’s and few of his contemporaries’ specimens have survived in Puerto Rico. -

A Courting Behavioral Study on a Hyacinth Macaw (Anodorhynchus Hyacinthinus) Pair
The Pegasus Review: UCF Undergraduate Research Journal (URJ) Volume 12 Issue 1 Article 2 2020 A Courting Behavioral Study on a Hyacinth Macaw (Anodorhynchus hyacinthinus) Pair Pamela Mulkay University of Central Florida Find similar works at: https://stars.library.ucf.edu/urj University of Central Florida Libraries http://library.ucf.edu This Article is brought to you for free and open access by the Office of Undergraduate Research at STARS. It has been accepted for inclusion in The Pegasus Review: UCF Undergraduate Research Journal (URJ) by an authorized editor of STARS. For more information, please contact [email protected]. Recommended Citation Mulkay, Pamela (2020) "A Courting Behavioral Study on a Hyacinth Macaw (Anodorhynchus hyacinthinus) Pair," The Pegasus Review: UCF Undergraduate Research Journal (URJ): Vol. 12 : Iss. 1 , Article 2. Available at: https://stars.library.ucf.edu/urj/vol12/iss1/2 Mulkay: A Courting Behavioral Study on a Hyacinth Macaw Published 9-17 Vol. 12.1: April 8, 2020 THE PEGASUS REVIEW: UNIVERSITY OF CENTRAL FLORIDA UNDERGRADUATE RESEARCH JOURNAL A Courting Behavioral Study on a Hyacinth Macaw (Anodorhynchus hyacinthinus) Pair By: Pamela Mulkay Faculty Mentor: Frank Logiudice UCF Department of Biology ABSTRACT: This study observes the courtship behaviors of an Anodorhynchus hyacinthinus pair in the Central Florida Zoo and Botanical Gardens in Sanford, Florida. A. hyacinthinus reproductive behaviors occur in four steps in the following order: Allopreening, Cloacal allopreening, Back to Back Copulation Position and finally, Copulation (Schneider 2006). Behavioral observations were taken twice a week for an average of 2 to 3 hours each day for ten weeks. The resulting data was analyzed based on the different actions, types of movement, and types of maintenance observed of the A. -

Pinho & Nogueira.Fm
ORNITOLOGIA NEOTROPICAL 14: 29–38, 2003 © The Neotropical Ornithological Society HYACINTH MACAW (ANODORHYNCHUS HYACINTHINUS) REPRODUCTION IN THE NORTHERN PANTANAL, MATO GROSSO, BRAZIL João Batista Pinho1 & Flávia M.B. Nogueira2 Instituto de Biociências, Universidade Federal de Mato Grosso, Av. Fernando Correa da Costa, s/n, 78060-900, Cuiabá, MT, Brazil. E-mail: [email protected] & [email protected] Resumo. – Reprodução de Arara Azul (Anodorhynchus hyacinthinus) no norte do Pantanal de Mato Grosso, Brasil. – A Arara Azul (Anodorhynchus hyacinthinus) é uma das muitas espécies da fauna bra- sileira que é ameaçada pela atividade humana, principalmente devido a perda de habitat. A população total com de cerca de 3.000 indivíduos de Arara Azul de vida livre ocorrem principalmente no Pantanal de Mato Grosso, Brasil, uma das maiores áreas alagadas do mundo. Queimadas para a manutenção das pastagens e o tráfico ilegal tem sido e ainda são as maiores ameaças para a sobrevivência da espécie no Pantanal. Nós estudamos as necessidades ambientais, o sucesso reprodutivo e aspectos da biologia reprodutiva da Arara Azul em uma área de 31.000 ha na região norte do Pantanal, de modo a aumentar as informações necessá- rias para elaboração de estratégias de manejo e conservação desta espécie. Procuramos por ninhos, marca- mos e medimos as cavidades dos ninhos e tentamos identificar o potencial de forrageamento e de sítios de nidificação que podem ser colonizados no futuro. Na área de estudo, as Araras Azuis usam cavidades de árvores para nidificar, em árvores entre 10–25 m de altura, a maioria nas bordas de matas. Catorze ninhos foram encontrados (0,045 ninho/100 ha), sendo 12 (85,7%) em apenas uma espécie de árvore, Sterculia ape- tala (Sterculiaceae). -

According to Dictionary
Extinction: The Parrots We’ve Lost By Desi Milpacher The definition of extinction is “the act or process of becoming extinct; a coming to an end or dying out: the extinction of a species.” Once extinction has been determined, there is usually no chance of a species recurring in a given ecosystem. In mankind’s active history of exploration, exploitation and settlement of new worlds, there has been much loss of natural resources. Parrots have suffered tremendously in this, with over twenty species having been permanently lost. And there are many more that are teetering on the edge, towards the interminable abyss. In this article we find out what happened to these lost treasures, learn which ones are currently being lost, and why this is important to our world. The Old and New Worlds and Their Lost Parrots Little is known of the natural history of most of the world’s extinct parrots, mainly because they disappeared before in-depth studies were conducted on them. It is generally believed, save the Central American macaws which were least known, that most fed on diets similar to today’s parrots (leaves, blossoms, seeds, nuts and fruits), frequented heavy forested areas and nested mainly in tree cavities. A number could not fly well, or were exceptionally tame, leading to their easy capture. Nearly all of these natural treasures vanished between the 18th and early 20th centuries, and the main reason for their loss was overhunting. Some lesser causes included egg collecting (popular with naturalists in the 19th century), diseases (introduced or endemic), drought, natural disasters, predation by introduced species, and habitat alternation. -

Caribbean Ornithology
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/333433561 The Baudin Expedition to Tenerife, St. Thomas, St. Croix, and Puerto Rico in 1796–1798 Article · May 2019 CITATION READS 1 201 2 authors: Justin J.F.J. Jansen Jérôme Fuchs Naturalis Biodiversity Center Muséum National d'Histoire Naturelle 125 PUBLICATIONS 179 CITATIONS 98 PUBLICATIONS 1,707 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Figuring out Steppe Whimbrel Numenius phaeopus alboaxillaris View project Threatened bird species of Polynesia and Melanesia View project All content following this page was uploaded by Justin J.F.J. Jansen on 31 May 2019. The user has requested enhancement of the downloaded file. The Journal of Caribbean Ornithology RESEARCH ARTICLE Vol. 32:39–48. 2019 The Baudin Expedition to Tenerife, St. Thomas, St. Croix, and Puerto Rico in 1796–1798 Justin J.F.J. Jansen Jérôme Fuchs Photo: Justin J.F.J. Jansen The Journal of Caribbean Ornithology www.birdscaribbean.org/jco ISSN 1544-4953 RESEARCH ARTICLE Vol. 32:39–48. 2019 www.birdscaribbean.org The Baudin Expedition to Tenerife, St. Thomas, St. Croix, and Puerto Rico in 1796–1798 Justin J.F.J. Jansen1 and Jérôme Fuchs2 Abstract The results of archival and collection research into the expedition led by Nicolas-Thomas Baudin in 1796–1798 to Tenerife, St. Thomas, St. Croix, and Puerto Rico are herein presented. The expedition brought home at least 296 specimens and was the first to collect in St. Thomas, St. -

The Baudin Expedition to Tenerife, St. Thomas, St
The Journal of Caribbean Ornithology RESEARCH ARTICLE Vol. 32:39–48. 2019 The Baudin Expedition to Tenerife, St. Thomas, St. Croix, and Puerto Rico in 1796–1798 Justin J.F.J. Jansen Jérôme Fuchs Photo: Justin J.F.J. Jansen The Journal of Caribbean Ornithology www.birdscaribbean.org/jco ISSN 1544-4953 RESEARCH ARTICLE Vol. 32:39–48. 2019 www.birdscaribbean.org The Baudin Expedition to Tenerife, St. Thomas, St. Croix, and Puerto Rico in 1796–1798 Justin J.F.J. Jansen1 and Jérôme Fuchs2 Abstract The results of archival and collection research into the expedition led by Nicolas-Thomas Baudin in 1796–1798 to Tenerife, St. Thomas, St. Croix, and Puerto Rico are herein presented. The expedition brought home at least 296 specimens and was the first to collect in St. Thomas, St. Croix, and Puerto Rico. Of these, at least 140 specimens still survive, the largest sin- gle-voyage collection from pre-1800 still available. Accounts of these specimens and those known to have vanished are present- ed here for the first time, adding to our knowledge of early Caribbean ornithology. The arguments of David K. Wetherbee (1985, 1986) that thefts by the Baudin expedition took place during a foray into Hispaniola are all shown to be suspect. Molecular and morphological work identified a Barn Owl Tyto( alba ssp) supposedly collected in Puerto Rico, thus providing the first possible documentation of the Barn Owl in Puerto Rico, but its exact taxonomic status remains unresolved. Our data thus cannot exclude the genuine Puerto Rican origin for this specimen. Keywords Barn Owl, Baudin, expedition, Puerto Rico, Tenerife, Tyto alba, Virgin Islands Resumen La expedición de Baudin a Tenerife, St.