Effects of Anisops Sardea (Hemiptera: Notonectidae) on Oviposition Habitat Selection by Mosquitoes and Other Dipterans and on Community Structure in Artificial Pools
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Venoms of Heteropteran Insects: a Treasure Trove of Diverse Pharmacological Toolkits
Review Venoms of Heteropteran Insects: A Treasure Trove of Diverse Pharmacological Toolkits Andrew A. Walker 1,*, Christiane Weirauch 2, Bryan G. Fry 3 and Glenn F. King 1 Received: 21 December 2015; Accepted: 26 January 2016; Published: 12 February 2016 Academic Editor: Jan Tytgat 1 Institute for Molecular Biosciences, The University of Queensland, St Lucia, QLD 4072, Australia; [email protected] (G.F.K.) 2 Department of Entomology, University of California, Riverside, CA 92521, USA; [email protected] (C.W.) 3 School of Biological Sciences, The University of Queensland, St Lucia, QLD 4072, Australia; [email protected] (B.G.F.) * Correspondence: [email protected]; Tel.: +61-7-3346-2011 Abstract: The piercing-sucking mouthparts of the true bugs (Insecta: Hemiptera: Heteroptera) have allowed diversification from a plant-feeding ancestor into a wide range of trophic strategies that include predation and blood-feeding. Crucial to the success of each of these strategies is the injection of venom. Here we review the current state of knowledge with regard to heteropteran venoms. Predaceous species produce venoms that induce rapid paralysis and liquefaction. These venoms are powerfully insecticidal, and may cause paralysis or death when injected into vertebrates. Disulfide- rich peptides, bioactive phospholipids, small molecules such as N,N-dimethylaniline and 1,2,5- trithiepane, and toxic enzymes such as phospholipase A2, have been reported in predatory venoms. However, the detailed composition and molecular targets of predatory venoms are largely unknown. In contrast, recent research into blood-feeding heteropterans has revealed the structure and function of many protein and non-protein components that facilitate acquisition of blood meals. -

Biological Control of Insect Pests in the Tropics - M
TROPICAL BIOLOGY AND CONSERVATION MANAGEMENT – Vol. III - Biological Control of Insect Pests In The Tropics - M. V. Sampaio, V. H. P. Bueno, L. C. P. Silveira and A. M. Auad BIOLOGICAL CONTROL OF INSECT PESTS IN THE TROPICS M. V. Sampaio Instituto de Ciências Agrária, Universidade Federal de Uberlândia, Brazil V. H. P. Bueno and L. C. P. Silveira Departamento de Entomologia, Universidade Federal de Lavras, Brazil A. M. Auad Embrapa Gado de Leite, Empresa Brasileira de Pesquisa Agropecuária, Brazil Keywords: Augmentative biological control, bacteria, classical biological control, conservation of natural enemies, fungi, insect, mite, natural enemy, nematode, predator, parasitoid, pathogen, virus. Contents 1. Introduction 2. Natural enemies of insects and mites 2.1. Entomophagous 2.1.1. Predators 2.1.2. Parasitoids 2.2. Entomopathogens 2.2.1. Fungi 2.2.2. Bacteria 2.2.3. Viruses 2.2.4. Nematodes 3. Categories of biological control 3.1. Natural Biological Control 3.2. Applied Biological Control 3.2.1. Classical Biological Control 3.2.2. Augmentative Biological Control 3.2.3. Conservation of Natural Enemies 4. Conclusions Glossary UNESCO – EOLSS Bibliography Biographical Sketches Summary SAMPLE CHAPTERS Biological control is a pest control method with low environmental impact and small contamination risk for humans, domestic animals and the environment. Several success cases of biological control can be found in the tropics around the world. The classical biological control has been applied with greater emphasis in Australia and Latin America, with many success cases of exotic natural enemies’ introduction for the control of exotic pests. Augmentative biocontrol is used in extensive areas in Latin America, especially in the cultures of sugar cane, coffee, and soybeans. -
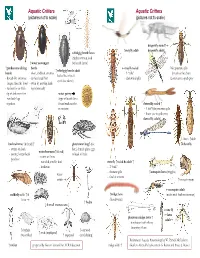
Aquatic Critters Aquatic Critters (Pictures Not to Scale) (Pictures Not to Scale)
Aquatic Critters Aquatic Critters (pictures not to scale) (pictures not to scale) dragonfly naiad↑ ↑ mayfly adult dragonfly adult↓ whirligig beetle larva (fairly common look ↑ water scavenger for beetle larvae) ↑ predaceous diving beetle mayfly naiad No apparent gills ↑ whirligig beetle adult beetle - short, clubbed antenna - 3 “tails” (breathes thru butt) - looks like it has 4 - thread-like antennae - surface head first - abdominal gills Lower jaw to grab prey eyes! (see above) longer than the head - swim by moving hind - surface for air with legs alternately tip of abdomen first water penny -row bklback legs (fbll(type of beetle larva together found under rocks damselfly naiad ↑ in streams - 3 leaf’-like posterior gills - lower jaw to grab prey damselfly adult↓ ←larva ↑adult backswimmer (& head) ↑ giant water bug↑ (toe dobsonfly - swims on back biter) female glues eggs water boatman↑(&head) - pointy, longer beak to back of male - swims on front -predator - rounded, smaller beak stonefly ↑naiad & adult ↑ -herbivore - 2 “tails” - thoracic gills ↑mosquito larva (wiggler) water - find in streams strider ↑mosquito pupa mosquito adult caddisfly adult ↑ & ↑midge larva (males with feather antennae) larva (bloodworm) ↑ hydra ↓ 4 small crustaceans ↓ crane fly ←larva phantom midge larva ↑ adult→ - translucent with silvery bflbuoyancy floats ↑ daphnia ↑ ostracod ↑ scud (amphipod) (water flea) ↑ copepod (seed shrimp) References: Aquatic Entomology by W. Patrick McCafferty ↑ rotifer prepared by Gwen Heistand for ACR Education midge adult ↑ Guide to Microlife by Kenneth G. Rainis and Bruce J. Russel 28 How do Aquatic Critters Get Their Air? Creeks are a lotic (flowing) systems as opposed to lentic (standing, i.e, pond) system. Look for … BREATHING IN AN AQUATIC ENVIRONMENT 1. -

Behavioral Plasticity to Risk of Predation: Oviposition Site Selection by a Mosquito in Response to Its Predators
8 Behavioral Plasticity to Risk of Predation: Oviposition Site Selection by a Mosquito in Response to its Predators Leon Blaustein1, 2 and Douglas W. Whitman3 1Community Ecology Laboratory, Institute of Evolution, Department of Evolutionary and Environmental Biology, Faculty of Sciences, University of Haifa, Haifa 31905, Israel. 2Center for Vector Biology, Rutgers University, New Brunswick, New Jersey 08901, USA. E-mail: [email protected] 34120 Department of Biological Sciences, Illinois State University, Normal, IL 61790, USA. E-mail: [email protected] Abstract Oviposition habitat selection in response to risk of predation is an environmentally induced phenotypic plastic response. We suggest predator- prey characteristics for which such a response is more likely to evolve: high vulnerability of progeny to the predator; deposition of all eggs from a single reproductive event in a single site (i.e., inability to spread the risk spatially); few opportunities to reproduce (i.e., unable to spread the risk temporally); during habitat assessment by the gravid female, high predictability of future risk of predation for the period in which the progeny develop at the site; the predator is common but some sites are predator-free. We summarize work done on a particular system—the mosquito Culiseta longiareolata Macquart and its predators in pool habitats, a likely candidate system for oviposition habitat selection in response to risk of predation given these proposed characteristics. Adult C. longiareolata females can chemically detect various predatory backswimmer species (Notonectidae) but do not appear to chemically detect odonate and urodele larvae. Evidence suggests however that ovipositing females may detect other predators by nonchemical cues. -

(Hemiptera-Heteroptera: Notonectidae) of the ORIENTAL REGION
Pacific Insects 10(2): 353-442 20 August 1968 THE ENITHARES (Hemiptera-Heteroptera: Notonectidae) OF THE ORIENTAL REGION By I. Lansbury HOPE DEPARTMENT OF ENTOMOLOGY, UNIVERSITY MUSEUM, OXFORD Abstract: This paper redescribes most of the species recorded from the Oriental Region. Keys to both sexes are given. Fifteen species and 1 subspecies are described for the first time. Five species are placed in synonymy and three previously described species have proved unrecognisable. This paper embodies the results of a study of the Oriental species of the genus Enithares. The main purpose being to collate the scattered descriptions and information concerning this genus. The geographical scope is limited to those species occurring east of the 60° of longitude. African, Mascarene and American species are excluded. No phylogenetic speculation is implied in any part of this paper. Wherever possible types have been examined in order to fix the species. In a few cases where types are no longer extant or available for study, I have utilized 'compared' specimens or the concept of the last reviewer. Full details are given under the relevant species. Acknowledgments: Many people have assisted in the preparation of this paper. In particular, I am deeply indebted to Dr G. Byers of the University of Kansas for making available to me a copy of G.T. Brooks unpublished thesis on Enithares. To Miss S. Na kata and Dr P. D. Ashlock of the Bishop Museum, Honolulu for the very large collection of un-named material sent to me. A glance at the location of many of the types of new species will show how valuable their contribution has been. -

Adult Nepidae of Florida
Graduate Student Project – Insect Classification – ENY 6166 University of Florida - Kendra Pesko - December 8, 2004 Adult Nepidae of Florida The family Nepidae, common name “waterscorpions”, is an aquatic insect family in the order Hemiptera (suborder Heteroptera). Of 13 species and three genera known throughout the United States and Canada, only five species in one genus (Ranatra) occur in Florida. Ranatra species are found in aquatic vegetation and debris, and can be collected by sweeping an aquatic net through vegetation along the edges of lakes. They will cling to emergent vegetation such as cattails to hide during the day, and return to the water surface at night. Ranatra species also make night time flights in order to colonize new areas, and will often end up on car windshields, which they may mistake for open water. Nepidae are unique among water bugs in possessing a stridulatory mechanism which consists of serrations on their fore-coxal cavity that contact coarse ridges which appear to be sclerotized setae. Both nymphs and adults of Ranatra possess these structures. Checklist of Species of Florida Ranatra Fabricius (Hemiptera: Heteroptera: Nepidae) R. australis Hungerford R. buenoi Hungerford R.drakei Hungerford R. kirkaldyi Torre-Bueno R. nigra Herrich-Schaeffer Key to Species of Adult Florida Nepidae (adapted from Sites and Polhemus 1994) 1. Prothorax with mid-ventral hollow groove (fig. 6)…Ranatra buenoi Hungerford 1’. Prothorax without mid-ventral hollow groove, but may be ventrally flattened or have a paired ventro-lateral longitudinal depressed lines .........................................................2 2. Penultimate antennal segment with lateral projection absent or if present, < ½ length of terminal antennal segment (Figs. -

Great Lakes Entomologist the Grea T Lakes E N Omo L O G Is T Published by the Michigan Entomological Society Vol
The Great Lakes Entomologist THE GREA Published by the Michigan Entomological Society Vol. 45, Nos. 3 & 4 Fall/Winter 2012 Volume 45 Nos. 3 & 4 ISSN 0090-0222 T LAKES Table of Contents THE Scholar, Teacher, and Mentor: A Tribute to Dr. J. E. McPherson ..............................................i E N GREAT LAKES Dr. J. E. McPherson, Educator and Researcher Extraordinaire: Biographical Sketch and T List of Publications OMO Thomas J. Henry ..................................................................................................111 J.E. McPherson – A Career of Exemplary Service and Contributions to the Entomological ENTOMOLOGIST Society of America L O George G. Kennedy .............................................................................................124 G Mcphersonarcys, a New Genus for Pentatoma aequalis Say (Heteroptera: Pentatomidae) IS Donald B. Thomas ................................................................................................127 T The Stink Bugs (Hemiptera: Heteroptera: Pentatomidae) of Missouri Robert W. Sites, Kristin B. Simpson, and Diane L. Wood ............................................134 Tymbal Morphology and Co-occurrence of Spartina Sap-feeding Insects (Hemiptera: Auchenorrhyncha) Stephen W. Wilson ...............................................................................................164 Pentatomoidea (Hemiptera: Pentatomidae, Scutelleridae) Associated with the Dioecious Shrub Florida Rosemary, Ceratiola ericoides (Ericaceae) A. G. Wheeler, Jr. .................................................................................................183 -

On Some Species of Heteroptera Collected in the Madeiran Islands
ACTA FAUNISTICA ENTOMOLOGICA MUSE! NATIONALIS PRAGAE Vol. 12, No. 117 Edit. 28. !1. 1987 (Acta [mm . enr. M us. Nat. Pragae, 12: 43-50) On some species of Heteroptera collected in the Madeiran Islands. By LlJDVIK H 0 BE R LAND T [Department of Entomology, National Museum { Nat. !-list.), Praha) Mr. Eric W. Classey submitted to me for identification Heteroptera collected by himself on the Madeiran Islands in 1962 and 1964. Al though during the last few years much has been done to enlarge the knowledge of the fauna of Heteroptera of Madeira it is still important to accummulate further or new records for these islands. The above mentioned material consists of 30 species, of which 5 species form new records for the fauna of Madeira. I am very grateful to Mr. E. W. Classey for entrusting me with the material for identification and for other invaluable help. C:ori x idae Corixa punctata ( rlliger, 1807) 2 99 and 1 nymph - Madeira: Ribeira de St. Luzia, 24. VIII. 1964. Distribution ,of this species is restricted rather to West Palaearctic. New record for Madeira. Only Corixa atfinis affinis Leach is recorded from Madeira, Canary Islancls ancl Azores. Sigara ( Vermicorixa) lateralis nakurui p,oisson, 1958 14 dd and 20 99 --- Porto santu: Ribeira da Serra do Oentro, 23 . VI. 1962. Sigara lateralis nakurui Poisson is recorded from Kenya (type locality l, Cape Verde Islands, Canary Islands and Madeira ( Lindberg 1961] . Notonectidae Anisops debilis canariensis Nol.ialhier, 1893 1 cl' - Madeira: S. Roque, 26. VI I f. 1964. Recorded from Canary Islands, Cape Verde Islandt:, South Morocco and Madeira (Lundblad 1949, Lindberg 1961), 43 Veliidae Velia maderensis Noualhier, 1897 2 nymphs of 2nd and 3rd instar - Madeira: Ribeira de St Luzia, 26. -

Acacia Flat Mite (Brevipalpus Acadiae Ryke & Meyer, Tenuipalpidae, Acarina): Doringboomplatmyt
Creepie-crawlies and such comprising: Common Names of Insects 1963, indicated as CNI Butterfly List 1959, indicated as BL Some names the sources of which are unknown, and indicated as such Gewone Insekname SKOENLAPPERLYS INSLUITENDE BOSLUISE, MYTE, SAAMGESTEL DEUR DIE AALWURMS EN SPINNEKOPPE LANDBOUTAALKOMITEE Saamgestel deur die MET MEDEWERKING VAN NAVORSINGSINSTITUUT VIR DIE PLANTBESKERMING TAALDIENSBURO Departement van Landbou-tegniese Dienste VAN DIE met medewerking van die DEPARTEMENT VAN ONDERWYS, KUNS EN LANDBOUTAALKOMITEE WETENSKAP van die Taaldiensburo 1959 1963 BUTTERFLY LIST Common Names of Insects COMPILED BY THE INCLUDING TICKS, MITES, EELWORMS AGRICULTURAL TERMINOLOGY AND SPIDERS COMMITTEE Compiled by the IN COLLABORATION WiTH PLANT PROTECTION RESEARCH THE INSTITUTE LANGUAGE SERVICES BUREAU Department of Agricultural Technical Services OF THE in collaboration with the DEPARTMENT OF EDUCATION, ARTS AND AGRICULTURAL TERMINOLOGY SCIENCE COMMITTEE DIE STAATSDRUKKER + PRETORIA + THE of the Language Service Bureau GOVERNMENT PRINTER 1963 1959 Rekenaarmatig en leksikografies herverwerk deur PJ Taljaard e-mail enquiries: [email protected] EXPLANATORY NOTES 1 The list was alphabetised electronically. 2 On the target-language side, ie to the right of the :, synonyms are separated by a comma, e.g.: fission: klowing, splyting The sequence of the translated terms does NOT indicate any preference. Preferred terms are underlined. 3 Where catchwords of similar form are used as different parts of speech and confusion may therefore -

Oviposition Responses of Two Mosquito Species to Pool Size and Predator Presence: Varying Trade- Offs Between Desiccation and Predation Risks
Israel Journal of Ecology & Evolution ISSN: 1565-9801 (Print) 2224-4662 (Online) Journal homepage: http://www.tandfonline.com/loi/tiee20 Oviposition responses of two mosquito species to pool size and predator presence: varying trade- offs between desiccation and predation risks Deborah Saward-Arav, Asaf Sadeh, Marc Mangel, Alan R. Templeton & Leon Blaustein To cite this article: Deborah Saward-Arav, Asaf Sadeh, Marc Mangel, Alan R. Templeton & Leon Blaustein (2016): Oviposition responses of two mosquito species to pool size and predator presence: varying trade-offs between desiccation and predation risks, Israel Journal of Ecology & Evolution, DOI: 10.1080/15659801.2015.1069113 To link to this article: http://dx.doi.org/10.1080/15659801.2015.1069113 Published online: 04 Aug 2016. Submit your article to this journal View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=tiee20 Download by: [63.249.84.85] Date: 04 August 2016, At: 05:20 Israel Journal of Ecology & Evolution, 2016 http://dx.doi.org/10.1080/15659801.2015.1069113 Oviposition responses of two mosquito species to pool size and predator presence: varying trade- offs between desiccation and predation risks Deborah Saward-Arav a, Asaf Sadeha, Marc Mangelb,c, Alan R. Templetona,d and Leon Blausteina* aCommunity Ecology Laboratory, Institute of Evolution and Department of Evolutionary and Environmental Biology, Faculty of Natural Sciences, University of Haifa, Haifa, Israel; bCenter for Stock Assessment Research and Department of Applied Mathematics and Statistics, Jack Baskin School of Engineering, University of California, Santa Cruz, CA, United States; cDepartment of Biology, University of Bergen, Bergen, Norway; dDepartment of Biology, Washington University, St. -

Common Backswimmer Notonecta Glauca (Linnaeus 1758) (Hemiptera: Notonectidae)1 Taryn B
EENY-738 Common Backswimmer Notonecta glauca (Linnaeus 1758) (Hemiptera: Notonectidae)1 Taryn B. Griffith and Jennifer L. Gillett-Kaufman2 Introduction Notonecta glauca, the common backswimmer (Figure 1), is an aquatic insect in the family Notonectidae. Insects in this family are commonly referred to as backswimmers or greater water boatman. Notonectids propel themselves through the water with their ventral side (belly) facing upwards, hence their common name of backswimmers (Figure 2). Notonectids can inflict wounds to humans with Figure 2. Notonecta sp. adult resting upside down underwater, which their proboscis (mouthpart), but this is very rare and often is typical of all Notonectids. is a result of rough handling. Credits: JRxpo. Flickr.com Distribution Although commonly collected in Europe (Soós et al. 2009), the common backswimmer can range from parts of northern Africa to western Siberia and northwestern China (Berchi 2013). Notonecta glauca is typically found in inland freshwater ponds, although they can be found in eutrophic (water excessively enriched in nutrients) freshwater bodies near the sea (Kjærstad et al. 2009). Many other Notonecta species occur in North America north of Mexico (Torre Bueno 1905). It is unclear if this species could become established if introduced to Florida, but its current distribu- tion includes several locations with similar climates and habitats. Figure 1. An adult Notonecta glauca (Linnaeus). Credits: David Nicholls 1. This document is EENY-738, one of a series of the Entomology and Nematology Department, UF/IFAS Extension. Original publication date August 2019. Visit the EDIS website at https://edis.ifas.ufl.edu for the currently supported version of this publication. -

Db Insect Guide Eng Compress
What are insects? How should I observe insects? Body Insects could be found everywhere- from flowers, divided into: shrubs, soil surface, to the sky and water! Observe carefully and you may discover them! Head You don’t need high-tech equipment to observe 3 pairs of legs Thorax insects. You’ll only need: Abdomen Eyes Camera Magnifier This card Safety rules during observation Insects are invertebrates with an Respect the nature. Do not harm any insects. estimated number of 30 million, forming 85% of world’s species Take away nothing but memories; leave nothing but footprints. Turn off the flashlight while taking photos to avoid disturbing the insects. ©February 2017 WWF-Hong Kong. All rights reserved. How to use this ID guide? Common species The purpose of this ID guide is to identify the in Hong Kong major groups of insects. An identification key English Name should be used to distinguish the species. Scientific Name In the classification system, we will divide organisms according to their body features. Insects belong to “Insec- ta” and are further divided into “orders”. Identify the insect group using the classification guide first, then use the colour coding to flip to the right section. Members of the order Common habitats of the order Characteristics of the Ways to distinguish insects insect order that are similar Behaviour and habits ©February 2017 WWF-Hong Kong. All rights reserved. Classification Guide Lepidoptera Odonata Hymenoptera Orthoptera e.g. butterfly, moth e.g. dragonfly, damselfly e.g. bee, wasp, ant e.g. grasshopper. katydid, cricket Compound eyes Compound eyes Strong Membranous Slender Membranous wings Leathery forewings, hind legs Wings covered with scales wings abdomen membranous hindwings Hemiptera Mantodea Diptera Coleoptera e.g.