Processing of Ceramic Materials---Lecture
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 Appalachian Center for Crafts Vince Pitelka, 2016 Glossary of Colored
Appalachian Center for Crafts Vince Pitelka, 2016 Glossary of Colored Clay Techniques Basalt Body: a clay body containing enough metallic oxides to fire to a gray-black or chocolate brown color. Basalt bodies may be fine-grained and smooth, as in Wedgewood black basalt ware, or may be grainy and rough, as in some sculptural works by Peter Voulkos and John Mason. Burnishing: historically the third stage in pottery decoration in almost every culture, after surface impressing and slip painting. Burnishing allows for a smooth, dense, glassy surface on any clay without the use of glazes. Color intensity is enhanced with burnishing almost as effectively as with the “wetting” effect of a clear glaze, and burnishing gives a satin texture achievable through no other method. Burnishing is generally done at the bone dry stage after re-introducing moisture into the clay with a thin coat of lard, glycerin, shortening, or cooking oil. One the lard or oil is absorbed, the surface is rubbed with a smooth hard object such as a shiny pebble or the back of a steel spoon. After firing, burnished wares are often polished further with pure tung-oil (a wood-finishing oil) or furniture polish. Clay Marquetry: similar to marquetry in wood. Small pieces of damp colored clays are assembled on a clay back-up slab (with a spray of vinegar water) to create pictorial imagery. Sometimes the designs are improvised during assembly, often overlapping components. For greater precision and pictorial clarity, an original design may be executed on poster-board, which is then cut up into individual templates. -
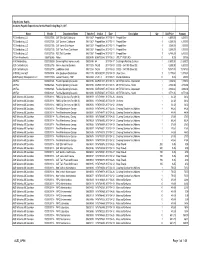
AUD AP96 Page 1 of 148 City of Cedar Rapids Accounts Payable Expenditures for the Week Ending May 23, 2017
City of Cedar Rapids Accounts Payable Expenditures for the Week Ending May 23, 2017 Name Vendor Department Name Voucher Invoice Date Description Qty Unit Price Amount 7G Distributiing LLC 0000027306 GLF Ellis Golf Clubhouse 00611267 Prepaid Beer 2017-05-11 Prepaid Beer 1 6,500.00 6,500.00 7G Distributiing LLC 0000027306 GLF Gardner Clubhouse 00611267 Prepaid Beer 2017-05-11 Prepaid Beer 1 6,500.00 6,500.00 7G Distributiing LLC 0000027306 GLF Jones Club House 00611267 Prepaid Beer 2017-05-11 Prepaid Beer 1 3,000.00 3,000.00 7G Distributiing LLC 0000027306 GLF Twin Pines Club House 00611267 Prepaid Beer 2017-05-11 Prepaid Beer 1 5,000.00 5,000.00 7G Distributiing LLC 0000027306 REC Tait Cummins 00611267 Prepaid Beer 2017-05-11 Prepaid Beer 1 6,300.00 6,300.00 A Shaw Investments 8359732346 Water 00008045 8359732346 2017-05-21 UTILITY REFUND 0 0.00 207.56 A1A Sandblasting 0000030259 Swimming Pool Improvements 00608746 44 2017-04-17 Caulking & Painting Services 1 21,890.00 21,890.00 AEC Contracting Inc 0000032798 Admin.-Housing Services 00611205 7428B 2017-05-03 CDBG - 384 19th Street SE 1 6,800.00 6,800.00 AEC Contracting Inc 0000032798 Lead Base Grant 00611204 7428A 2017-05-03 CDBG - 384 19th Street SE 1 19,747.80 19,747.80 AFSCME Local 620 0000000154 Misc Employee Deductions 00611135 AFSCME051 2017-05-19 Union Dues 1 11,175.63 11,175.63 AKM Property Management LLC 0000017300 Leased Housing - HAP 00328290 V1251-1 2017-05-01 Rental Assistance 0 0.00 258.00 ASI Flex 0000021324 Flexible Spending Accounts 00610736 ASIF051517 2017-05-15 2017 FSA -

Thematic Manifestations: an Aesthetic Journey. Jeff Kise East Tennessee State University
East Tennessee State University Digital Commons @ East Tennessee State University Electronic Theses and Dissertations Student Works 5-2004 Thematic Manifestations: an Aesthetic Journey. Jeff Kise East Tennessee State University Follow this and additional works at: https://dc.etsu.edu/etd Part of the Art and Design Commons Recommended Citation Kise, Jeff, "Thematic Manifestations: an Aesthetic Journey." (2004). Electronic Theses and Dissertations. Paper 877. https://dc.etsu.edu/ etd/877 This Thesis - Open Access is brought to you for free and open access by the Student Works at Digital Commons @ East Tennessee State University. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons @ East Tennessee State University. For more information, please contact [email protected]. Thematic Manifestations: an Aesthetic Journey ______________________ A thesis presented to the faculty of the Department of Art and Design East Tennessee State University ______________________ In partial fulfillment of the requirements for the degree of Master of Fine Arts in Studio Art ______________________ by Jeff Kise May 2004 _____________________ Don Davis, Chair Anita DeAngelis Catherine Murray Keywords: Ceramics, Simplicity, Aesthetics, Saggar Firing, Flash Firing, Naked Raku ABSTRACT Thematic Manifestations: An Aesthetic Journey by Jeff Kise This thesis, in support of the Master of Fine Arts exhibition entitled Thematic Manifestations at East Tennessee State University, Carroll Reece Museum, Johnson City, Tennessee, March 2-12, 2004, describes in detail three aesthetic themes that are manifested in the work exhibited. The artist discusses his journey in establishing a “criterion of aesthetic values” whereby his work is conceptually developed. The three themes – The Paradox of Simplicity, The Decorative Power of Nature, and The Beauty of the Irregular – are founded on historical and contemporary influences and are further described in practical application of form and process. -

Bisque Or Biscuit: the First Firing a Pot Undergoes to Prepare It for Glazing
Glossary of Ceramic Terms: Introduction to Ceramic Terms and Definitions Bisque or Biscuit: The first firing a pot undergoes to prepare it for glazing. In industry this is done at a higher temperature than the subsequent glaze firing, the reverse is usually true of the studio potter. Burnishing: A technique where the Leather hard clay is polished with a hard instrument to force the smallest clay particles to the surface creating a soft sheen. This surface remains after the pot is fired so long as the firing temperature is kept below 1100oC. Clay: AL2O3 2SiO2 2H2O. The decomposition of Granite through the process of Kaolinization creates clay (see Kaolinization). Clay is a mineral with a plate (platelet) like structure; it is these plates, (about 0.5 microns across) when lubricated with water, slide against each other to form the plastic mass we know as clay (see Water). 'Primary ' clays are those found close to the area of Kaolinization and hence the purest (Kaolin or China Clays). Secondary clays are those moved by water away form the site of Kaolinization and get progressively more plastic and less pure (Ball Clays, Fire clays, Earthenwares). Clay Body: A clay designed for a special purpose. It is created by blending different clays of by adding to clays other materials, such as feldspar and flint in order to produce a desired workability, maturing temperature, or finished result. A clay body is the result of mans technology. Cobalt: One of the strongest coloring oxides used by the potter. Cobalt creates a dark dense royal blue in most cases. -

Ceramics Monthly Apr04 Cei04
editor Sherman Hall associate editor Tim Frederich assistant editor Renee Fairchild design Paula John production manager John Wilson production specialist David Houghton advertising manager Steve Hecker advertising assistant Debbie Plummer circulation manager Cleo Eddie publisher Marcus Bailey editorial, advertising and circulation offices 735 Ceramic Place Westerville, Ohio 43081 USA telephone editorial: (614) 895-4213 advertising: (614) 794-5809 classifieds: (614) 895-4212 customer service: (614) 794-5890 fax (614) 891-8960 e-mail [email protected] [email protected] [email protected] [email protected] website www.ceramicsmonthly.org Ceramics Monthly (ISSN 0009-0328) is published monthly, except July and August, by The American Ceramic Society, 735 Ceramic Place, Westerville, Ohio 43081; www.ceramics.org. Periodicals postage paid at Westerville, Ohio, and additional mailing offices. Opinions expressed are those of the contributors and do not necessarily represent those of the editors or The Ameri can Ceramic Society. subscription rates: One year $32, two years $60, three years $86. Add $25 per year for subscriptions outside North America. In Canada, add GST (registration number R123994618). change of address: Please give us four weeks advance notice. Send the magazine address label as well as your new address to: Ceramics Monthly, Circulation De partment, PO Box 6136, Westerville, OH 43086-6136. contributors: Writing and photographic guidelines are available on request. Send manuscripts and visual sup port (slides, transparencies, photographs, drawings, etc.) to Ceramics Monthly, 735 Ceramic PI., Westerville, OH 43081. We also accept unillustrated texts e-mailed to [email protected] or faxed to (614) 891-8960. indexing: An index of each year's feature articles appears in the December issue. -

Archaeological and Historic Pottery Production Sites Guidelines for Best Practice Summary
Archaeological and Historic Pottery Production Sites Guidelines for Best Practice Summary This document provides practical guidance on how to investigate sites where pottery production has taken place. It describes how to anticipate and locate pottery production sites and the types of evidence that may be found. This document also provides advice on the available methods and strategies for examining, recording and sampling features and finds of various types at each stage of the work. The different techniques for establishing the date of pottery production, and for characterising the products of a site, are given particular emphasis. This document was compiled by Harriet White, Sarah Paynter and Duncan Brown with contributions by Joanne Best, Chris Cumberpatch, David Dawson, Peter Ellis, Jane Evans, Laurence Jones, Oliver Kent, Gareth Perry, The Prince’s Regeneration Trust, Ian Roberts, Kerry Tyler and Ann Woodward. It is one of four Historic England publications concerning industrial processes in the past, the other three being: ■ Science for Historic Industries: Guidelines for the Investigation of 17th- to 19th- Century Industries ■ Archaeological Evidence for Glassworking: Guidelines for Best Practice ■ Archaeometallurgy: Guidelines for Best Practice. First published by Historic England October 2015. All images © Historic England unless otherwise stated. www.historicengland.org.uk/advice/technical-advice/archaeological-science/ Front cover images: clockwise from top left Reconstruction of a 17th-century kiln from Potters A 14th-century -

The Process of Pit Firing
The Process of Pit Firing Introduction My interest in pit fired pottery comes from my desire to strip back the complexities of life and to demonstrate the essential processes involved in the production of pottery. So much of our lives is surrounded by mass production that is epitomised by catalogues of mass produced wares from Asia and these wares can be bought at very cheap prices from homewares shops . These items are functional, well produced but lack character or expression of individuality that would make them objects of art. Early pottery has been found in places such as Jomon, Japan (10,500 BC), but the earliest ceramic objects like the Venus of Dolní Věstonice figurine discovered in the Czech Republic, date back to around 25,000 BC, and pottery vessels from Jiangxi, China date back to 20,000 BC. The earliest pottery probably developed by accident and there are two main theories of development. It may have been from observing how the earth became baked around fire pits or it may have come from the accidental burning of clay-lined baskets. The clay was used to line baskets made of natural fibre in order prevent loss of grains or seeds that were stored in such baskets. Pottery developed in response to needs and so pots had a purpose. This included storing grains or flour, carting, cooking pots, ritual and funeral rites. The rulers of Egypt had their internal organs mummified in canopic jars. The first use of pottery vessels for storing water and food is probably around 9000 or 10,000 BC and so pottery is one of man’s most ancient industries. -

DAACS Cataloging Manual: Ceramics
DAACS Cataloging Manual: Ceramics by Jennifer Aultman, Nick Bon-Harper, Leslie Cooper, Jillian Galle, Kate Grillo, and Karen Smith OCTOBER 2003 LAST UPDATED OCTOBER 2014 INTRODUCTION .......................................................................................................... 6 1. CERAMIC ARTIFACT ENTRY ...................................................................................... 7 1.1 COUNT ......................................................................................................................... 7 1.2 WARE .......................................................................................................................... 8 1.3 MATERIAL ..................................................................................................................... 8 1.4 MANUFACTURING TECHNIQUE .......................................................................................... 9 1.5 VESSEL CATEGORY .......................................................................................................... 9 1.6 FORM ......................................................................................................................... 10 1.6.1 Teaware ............................................................................................................ 10 1.6.2 Tableware ......................................................................................................... 11 1.6.3 Utilitarian ......................................................................................................... -

Decorating Techniques
ceramic artsdaily.org three great pottery decorating techniques a guide to sgraffito, how to make and use terra sigillata, and creating and coloring highly textured surfaces www.ceramicartsdaily.org | Copyright © 2010, Ceramic Publications Company | Three Great Pottery Decorating Techniques | i Three Great Pottery Decorating Techniques A guide to sgraffito, how to make and use terra sigillata, and creating and coloring highly textured surfaces Decorating your work before it’s fired provides you with a lot of creative opportunities. At the soft clay stage, you can stamp and texture your clay using many types and kinds of objects. When the clay is leather hard, you can coat your work with colored slips and carve away to create patterns. And when your clay is bone dry, you can apply terra sigillata and burnish the surface to a high sheen. Scratching the Surface: A Step-by-Step Guide to Sgraffito by Wayne Bates Sgraffito comes from the Italian word graffito meaning “to scratch,” and Wayne Bates does more than scratch the surface with this informative tutorial on the tips and techniques of getting the best results. Using both cutting and scratching techniques, he demonstrates the finer points of line work, scraping large areas and cross-hatching. If you’re into making your own glazes, he’s provided 19 colorful engobes along with 5 recipes for various clear glazes you can try out. Burnishing with Terra Sigillata by Sumi von Dassow Terra sigillata means ‘sealed earth’ and comes from the name of a type of Roman pottery mass-produced around the first century AD. -

Super-Refined Terra Sigillata Vince Pitelka, ©2019 Note
Super-Refined Terra Sigillata Vince Pitelka, ©2019 Note: This article is offered free to everyone, with the clear understanding that it cannot be copied for sale under any circumstance, and can be copied for distribution only with attribution to and permission of the author. Introduction Traditionally, the primary objective of terra sigillata refined slip coatings is to get a high satin gloss without a glaze, using simple, time-effective polishing methods. There is some misunderstanding about the translation of the term, and many people believe that it means “clay with a sealed surface.” The translation is actually closer to “clay with little images,” referring to the finely-detailed raised-relief imagery on Roman Arretine ware. Arretine ware always featured a terra sigillata finish and represented the pinnacle of Roman ceramics, and the term "terra sigillata" evolved to apply to the refined slip itself used on any sort of ware. Super-refined polishing slips were first used in the Middle East at least 1000 BC, and reached a pinnacle of aesthetics and technical accomplishment with the terra sigillatas used on Greek and Roman pottery. The purity of terra sigillata is perhaps most celebrated on fine Roman Arretine wares, with the monochromatic red high-gloss coating emphasizing the complex relief decoration mentioned above. But like so many innovations of the Romans, the technology was completely lost in the early middle ages. Most of the popular published terra sigillata recipes do not separate and eliminate the large particles adequately, and the product is not a true terra sig. In the late 1980s I began researching terra sigillatas in conjunction with the Ancient Clay classes and workshops I developed at UMass-Amherst. -

The Goal of This Document Is to Lead a Future Cataloger Through the Decisions Made and Issues Encountered in Cataloging Common
DAACS Cataloging Manual: Ceramics by Jennifer Aultman, Nick Bon-Harper, Leslie Cooper, Jillian Galle, Kate Grillo, and Karen Smith OCTOBER 2003 UPDATED OCTOBER 2013 1 INTRODUCTION.................................................................................................... 5 1. MAIN CERAMICS TABLE ........................................................................ 6 1.1 Count .............................................................................................................. 6 1.2 Material.......................................................................................................... 6 1.3 Manufacturing Technique .............................................................................. 7 1.4 Ware ............................................................................................................... 8 1.5 Vessel Category ............................................................................................. 8 1.6 Form ............................................................................................................... 8 1.7 Mended Form ............................................................................................... 10 1.8 Completeness ............................................................................................... 11 1.9 Paste Color .................................................................................................. 11 1.10 Exterior Surface ........................................................................................... 11 -

Mold Making Techniques | Third Edition |
ceramic artsdaily.org ceramic mold making techniques | Third Edition | tips for making plaster, bisque, and styrofoam molds, making and using casting slip, and decorating ceramic surfaces This special report is brought to you with the support of Evans Ceramic Supply Ceramic Mold Making Techniques | Third Edition Tips for Making Plaster, Bisque, and Styrofoam Molds, Making and Using Casting Slip, and Decorating Ceramic Surfaces Molds make it possible to repeat patterns and forms for a variety of reasons. For thousands of years, potters have used molds both for forming and decorating and often both have been accomplished at the same time. Whether you choose to try press molds with slabs of clay or slip-casting molds for slip-cast pieces, you’ll discover that molds provide a way to create uniform pieces that can save you time and provide you with the means to concentrate on surface decorations. How to Make a Model and a Mold for Slip-casting by Andrew Gilliatt Learn how to make a model for a shape that you create a mold from. Whether simple or complex, you’ll need to follow the same steps for figuring out to make an original model then determining the best way to cast the mold pieces. How to Make and Apply Decals to Slip-cast Vessels by Linda Gates One of the advantages of slip-cast work is the smooth surface, which is perfect for adding decals. Linda Gates shows you how to slip-cast smooth pieces then how to create decals and apply them. Making a Tile Press Mold by Ursula Hargens Ursula Hargens was looking for larger surfaces to decorate so she came up with a tile design that suited her needs.