Super-Refined Terra Sigillata Vince Pitelka, ©2019 Note
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

9. Ceramic Arts
Profile No.: 38 NIC Code: 23933 CEREMIC ARTS 1. INTRODUCTION: Ceramic art is art made from ceramic materials, including clay. It may take forms including art ware, tile, figurines, sculpture, and tableware. Ceramic art is one of the arts, particularly the visual arts. Of these, it is one of the plastic arts. While some ceramics are considered fine art, some are considered to be decorative, industrial or applied art objects. Ceramics may also be considered artifacts in archaeology. Ceramic art can be made by one person or by a group of people. In a pottery or ceramic factory, a group of people design, manufacture and decorate the art ware. Products from a pottery are sometimes referred to as "art pottery".[1] In a one-person pottery studio, ceramists or potters produce studio pottery. Most traditional ceramic products were made from clay (or clay mixed with other materials), shaped and subjected to heat, and tableware and decorative ceramics are generally still made this way. In modern ceramic engineering usage, ceramics is the art and science of making objects from inorganic, non-metallic materials by the action of heat. It excludes glass and mosaic made from glass tesserae. There is a long history of ceramic art in almost all developed cultures, and often ceramic objects are all the artistic evidence left from vanished cultures. Elements of ceramic art, upon which different degrees of emphasis have been placed at different times, are the shape of the object, its decoration by painting, carving and other methods, and the glazing found on most ceramics. 2. -

1 Appalachian Center for Crafts Vince Pitelka, 2016 Glossary of Colored
Appalachian Center for Crafts Vince Pitelka, 2016 Glossary of Colored Clay Techniques Basalt Body: a clay body containing enough metallic oxides to fire to a gray-black or chocolate brown color. Basalt bodies may be fine-grained and smooth, as in Wedgewood black basalt ware, or may be grainy and rough, as in some sculptural works by Peter Voulkos and John Mason. Burnishing: historically the third stage in pottery decoration in almost every culture, after surface impressing and slip painting. Burnishing allows for a smooth, dense, glassy surface on any clay without the use of glazes. Color intensity is enhanced with burnishing almost as effectively as with the “wetting” effect of a clear glaze, and burnishing gives a satin texture achievable through no other method. Burnishing is generally done at the bone dry stage after re-introducing moisture into the clay with a thin coat of lard, glycerin, shortening, or cooking oil. One the lard or oil is absorbed, the surface is rubbed with a smooth hard object such as a shiny pebble or the back of a steel spoon. After firing, burnished wares are often polished further with pure tung-oil (a wood-finishing oil) or furniture polish. Clay Marquetry: similar to marquetry in wood. Small pieces of damp colored clays are assembled on a clay back-up slab (with a spray of vinegar water) to create pictorial imagery. Sometimes the designs are improvised during assembly, often overlapping components. For greater precision and pictorial clarity, an original design may be executed on poster-board, which is then cut up into individual templates. -
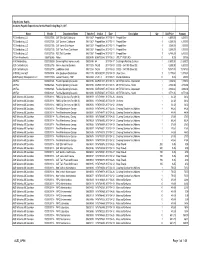
AUD AP96 Page 1 of 148 City of Cedar Rapids Accounts Payable Expenditures for the Week Ending May 23, 2017
City of Cedar Rapids Accounts Payable Expenditures for the Week Ending May 23, 2017 Name Vendor Department Name Voucher Invoice Date Description Qty Unit Price Amount 7G Distributiing LLC 0000027306 GLF Ellis Golf Clubhouse 00611267 Prepaid Beer 2017-05-11 Prepaid Beer 1 6,500.00 6,500.00 7G Distributiing LLC 0000027306 GLF Gardner Clubhouse 00611267 Prepaid Beer 2017-05-11 Prepaid Beer 1 6,500.00 6,500.00 7G Distributiing LLC 0000027306 GLF Jones Club House 00611267 Prepaid Beer 2017-05-11 Prepaid Beer 1 3,000.00 3,000.00 7G Distributiing LLC 0000027306 GLF Twin Pines Club House 00611267 Prepaid Beer 2017-05-11 Prepaid Beer 1 5,000.00 5,000.00 7G Distributiing LLC 0000027306 REC Tait Cummins 00611267 Prepaid Beer 2017-05-11 Prepaid Beer 1 6,300.00 6,300.00 A Shaw Investments 8359732346 Water 00008045 8359732346 2017-05-21 UTILITY REFUND 0 0.00 207.56 A1A Sandblasting 0000030259 Swimming Pool Improvements 00608746 44 2017-04-17 Caulking & Painting Services 1 21,890.00 21,890.00 AEC Contracting Inc 0000032798 Admin.-Housing Services 00611205 7428B 2017-05-03 CDBG - 384 19th Street SE 1 6,800.00 6,800.00 AEC Contracting Inc 0000032798 Lead Base Grant 00611204 7428A 2017-05-03 CDBG - 384 19th Street SE 1 19,747.80 19,747.80 AFSCME Local 620 0000000154 Misc Employee Deductions 00611135 AFSCME051 2017-05-19 Union Dues 1 11,175.63 11,175.63 AKM Property Management LLC 0000017300 Leased Housing - HAP 00328290 V1251-1 2017-05-01 Rental Assistance 0 0.00 258.00 ASI Flex 0000021324 Flexible Spending Accounts 00610736 ASIF051517 2017-05-15 2017 FSA -

Thematic Manifestations: an Aesthetic Journey. Jeff Kise East Tennessee State University
East Tennessee State University Digital Commons @ East Tennessee State University Electronic Theses and Dissertations Student Works 5-2004 Thematic Manifestations: an Aesthetic Journey. Jeff Kise East Tennessee State University Follow this and additional works at: https://dc.etsu.edu/etd Part of the Art and Design Commons Recommended Citation Kise, Jeff, "Thematic Manifestations: an Aesthetic Journey." (2004). Electronic Theses and Dissertations. Paper 877. https://dc.etsu.edu/ etd/877 This Thesis - Open Access is brought to you for free and open access by the Student Works at Digital Commons @ East Tennessee State University. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons @ East Tennessee State University. For more information, please contact [email protected]. Thematic Manifestations: an Aesthetic Journey ______________________ A thesis presented to the faculty of the Department of Art and Design East Tennessee State University ______________________ In partial fulfillment of the requirements for the degree of Master of Fine Arts in Studio Art ______________________ by Jeff Kise May 2004 _____________________ Don Davis, Chair Anita DeAngelis Catherine Murray Keywords: Ceramics, Simplicity, Aesthetics, Saggar Firing, Flash Firing, Naked Raku ABSTRACT Thematic Manifestations: An Aesthetic Journey by Jeff Kise This thesis, in support of the Master of Fine Arts exhibition entitled Thematic Manifestations at East Tennessee State University, Carroll Reece Museum, Johnson City, Tennessee, March 2-12, 2004, describes in detail three aesthetic themes that are manifested in the work exhibited. The artist discusses his journey in establishing a “criterion of aesthetic values” whereby his work is conceptually developed. The three themes – The Paradox of Simplicity, The Decorative Power of Nature, and The Beauty of the Irregular – are founded on historical and contemporary influences and are further described in practical application of form and process. -

Bisque Or Biscuit: the First Firing a Pot Undergoes to Prepare It for Glazing
Glossary of Ceramic Terms: Introduction to Ceramic Terms and Definitions Bisque or Biscuit: The first firing a pot undergoes to prepare it for glazing. In industry this is done at a higher temperature than the subsequent glaze firing, the reverse is usually true of the studio potter. Burnishing: A technique where the Leather hard clay is polished with a hard instrument to force the smallest clay particles to the surface creating a soft sheen. This surface remains after the pot is fired so long as the firing temperature is kept below 1100oC. Clay: AL2O3 2SiO2 2H2O. The decomposition of Granite through the process of Kaolinization creates clay (see Kaolinization). Clay is a mineral with a plate (platelet) like structure; it is these plates, (about 0.5 microns across) when lubricated with water, slide against each other to form the plastic mass we know as clay (see Water). 'Primary ' clays are those found close to the area of Kaolinization and hence the purest (Kaolin or China Clays). Secondary clays are those moved by water away form the site of Kaolinization and get progressively more plastic and less pure (Ball Clays, Fire clays, Earthenwares). Clay Body: A clay designed for a special purpose. It is created by blending different clays of by adding to clays other materials, such as feldspar and flint in order to produce a desired workability, maturing temperature, or finished result. A clay body is the result of mans technology. Cobalt: One of the strongest coloring oxides used by the potter. Cobalt creates a dark dense royal blue in most cases. -

Ancient Lamps in the J. Paul Getty Museum
ANCIENT LAMPS THE J. PAUL GETTY MUSEUM Ancient Lamps in the J. Paul Getty Museum presents over six hundred lamps made in production centers that were active across the ancient Mediterranean world between 800 B.C. and A.D. 800. Notable for their marvelous variety—from simple clay saucers GETTYIN THE PAUL J. MUSEUM that held just oil and a wick to elaborate figural lighting fixtures in bronze and precious metals— the Getty lamps display a number of unprecedented shapes and decors. Most were made in Roman workshops, which met the ubiquitous need for portable illumination in residences, public spaces, religious sanctuaries, and graves. The omnipresent oil lamp is a font of popular imagery, illustrating myths, nature, and the activities and entertainments of daily life in antiquity. Presenting a largely unpublished collection, this extensive catalogue is ` an invaluable resource for specialists in lychnology, art history, and archaeology. Front cover: Detail of cat. 86 BUSSIÈRE AND LINDROS WOHL Back cover: Cat. 155 Jean Bussière was an associate researcher with UPR 217 CNRS, Antiquités africaines and was also from getty publications associated with UMR 140-390 CNRS Lattes, Ancient Terracottas from South Italy and Sicily University of Montpellier. His publications include in the J. Paul Getty Museum Lampes antiques d'Algérie and Lampes antiques de Maria Lucia Ferruzza Roman Mosaics in the J. Paul Getty Museum Méditerranée: La collection Rivel, in collaboration Alexis Belis with Jean-Claude Rivel. Birgitta Lindros Wohl is professor emeritus of Art History and Classics at California State University, Northridge. Her excavations include sites in her native Sweden as well as Italy and Greece, the latter at Isthmia, where she is still active. -

Ceramics Monthly Apr04 Cei04
editor Sherman Hall associate editor Tim Frederich assistant editor Renee Fairchild design Paula John production manager John Wilson production specialist David Houghton advertising manager Steve Hecker advertising assistant Debbie Plummer circulation manager Cleo Eddie publisher Marcus Bailey editorial, advertising and circulation offices 735 Ceramic Place Westerville, Ohio 43081 USA telephone editorial: (614) 895-4213 advertising: (614) 794-5809 classifieds: (614) 895-4212 customer service: (614) 794-5890 fax (614) 891-8960 e-mail [email protected] [email protected] [email protected] [email protected] website www.ceramicsmonthly.org Ceramics Monthly (ISSN 0009-0328) is published monthly, except July and August, by The American Ceramic Society, 735 Ceramic Place, Westerville, Ohio 43081; www.ceramics.org. Periodicals postage paid at Westerville, Ohio, and additional mailing offices. Opinions expressed are those of the contributors and do not necessarily represent those of the editors or The Ameri can Ceramic Society. subscription rates: One year $32, two years $60, three years $86. Add $25 per year for subscriptions outside North America. In Canada, add GST (registration number R123994618). change of address: Please give us four weeks advance notice. Send the magazine address label as well as your new address to: Ceramics Monthly, Circulation De partment, PO Box 6136, Westerville, OH 43086-6136. contributors: Writing and photographic guidelines are available on request. Send manuscripts and visual sup port (slides, transparencies, photographs, drawings, etc.) to Ceramics Monthly, 735 Ceramic PI., Westerville, OH 43081. We also accept unillustrated texts e-mailed to [email protected] or faxed to (614) 891-8960. indexing: An index of each year's feature articles appears in the December issue. -

Late Hellenistic to Mediaeval Fine Wares of the Aegean Coast of Anatolia Prace Instytutu Kultur Śródziemnomorskich I Orientalnych Polskiej Akademii Nauk Tom 1
LATE HELLENISTIC TO MEDIAEVAL FINE WARES OF THE AEGEAN COAST OF ANATOLIA PRACE INSTYTUTU KULTUR ŚRÓDZIEMNOMORSKICH I ORIENTALNYCH POLSKIEJ AKADEMII NAUK TOM 1 CERAMIKA STOŁOWA EGEJSKIEGO WYBRZEŻA ANATOLII OD OKRESU PÓŹNOHELLENISTYCZNEGO DO ŚREDNIOWIECZA PRODUKCJA, NAŚLADOWNICTWA I ZASTOSOWANIE pod redakcją HENRYKA MEYZA przy współpracy KRZYSZTOFA DOMŻALSKIEGO WYDAWNICTWO NERITON Warszawa 2014 TRAVAUX DE L’INSTITUT DES CULTURES MÉDITERRANÉENNES ET ORIENTALES DE L’ACADÉMIE POLONAISE DES SCIENCES TOME 1 LATE HELLENISTIC TO MEDIAEVAL FINE WARES OF THE AEGEAN COAST OF ANATOLIA THEIR PRODUCTION, IMITATION AND USE édité par HENRYK MEYZA avec la collaboration de KRZYSZTOF DOMŻALSKI ÉDITIONS NERITON Varsovie 2014 Contents Introduction . 7 PART I: Production at Pergamon and its region Sigillata of Pergamon – Eastern Sigillata C. Problems of classifi cation and chronology – by S. Japp, Deutsches Archäologisches Institut, Berlin . 11 La production des ateliers de céramique de Pergame (Vallée de Ketios) : un aperçu général – by O. Bounegru, Catedra de Istorie Veche şi Arheologie Facultatea de Istorie. Universitatea „Al. I. Cuza” Iaşi . 23 Statistical interpretation of elemental concentration data and the origin of Pergamene pottery – by H. Mommsen, Helmholtz-Institut für Strahlen- und Kernphysik, Rheinische Friedrich- Wilhelms-Universität Bonn & S. Japp, Deutsches Archäologisches Institut, Berlin . 31 A Pergamene (?) modiolus in the National Museum of Denmark – by J. Lund, Antiksamlingen, Nationalmuseet, København . 41 PART II: Other production sites of western Anatolia Ephesus – local vs import: Th e Early Byzantine fi ne ware – by A. Waldner, Institut für Kulturgeschichte der Antike, Österreichische Akademie der Wissenschaft en & S. Ladstätter, Österreichisches Archäologisches Institut, Wien . 49 Microstructural characteristics of appliqué wares from Ephesus – by L. Peloschek, Österreichisches Archäologisches Institut, Wien & A. -

Pisan Sigillata Augustan Ideology with a Few Images
PISAN SIGILLATA Augustan ideology with a few images Simonetta Menchelli – Paolo Sangriso Abstract In Italy, after Arezzo, Pisa was the main production centre of terra sigillata. Although the city undoubtedly espoused Augustan policies and its potters were deeply ingrained with the Imperial ideology, the decorated vessels were produced only in small numbers, (about 3 %) and the Augustan subjects, were even more scarce, despite being familiar to the Pisan artisans (for example the cycle of Heracles and Omphale, the She-Wolf and the Twins). In our opinion the Pisan workshops did not need to display the motifs of Augustan propaganda on their vases because of the city’s proven loyalty to Octavianus: and its elites had been Rome’s faithful allies for a long time, most probably thanks to the role played by Maecenas. Hence, Cn. Ateius and the other Pisan potters could give their full attention to the economic aspects of the sigillata productions, without being concerned about Augustan propaganda. Therefore, they specialized in plain vessels most probably because they could be easily piled together and were much more convenient for transport and trade than decorated chalices and large cups. Their market strategy, combining mass production and distribution, proved to be successful throughout the Roman Empire and beyond. The various forms of Augustan propaganda development that derived from the transforma- permeated all the aspects of social life through an tions in Roman society following the period of the astute and widespread exploitation of all the avail- triumvirate. able means of communication. The Roman world After Arezzo, Pisa was the main production cen- was a world of images and it was through these tre where terra sigillata workshops were concen- that the Imperial ideology penetrated the different trated in the city’s Northern suburbium and in the social classes also through objects of daily use such territory along the Auser/Auserculus rivers (Fig. -

CHAPTER XI Pottery. Terra Sigillata Or Samian Ware
CHAPTER XI Pottery. Terra Sigillata or Samian Ware OF all the characteristic remains found on Roman sites there is nothing more enduring than the shards of broken pottery. Colours, textures, shapes are usually preserved, and thus it is often possible to reconstruct from one or two tiny fragments the exact form of vessels long since hopelessly broken and scattered. These shards, too, as more facts are ascertained about them, become increasingly valuable as a means of fixing dates. Among the Romans, as among ourselves, shapes and methods of decoration changed with the fashions of the time, and, if we could get a thorough knowledge of such changes, we should have a chronological series even more valuable than coins to the archaeologist. In Britain little has as yet been done to achieve such a result. We have indeed in our island few sites which were first occupied sufficiently late, or were abandoned sufficiently early, to be helpful. Cemeteries in which the interments can be dated with any certainty are no less rare. Sites of towns like Silchester were occupied for a long period, and in these stratification is, as a rule, difficult. But we may hope something from the careful records made at Manchester, and from the excavations now proceeding at Corbridge-on-Tyne and Caersws. On the Continent the study of the subject is more happily placed. The various stages that marked the advance of the Roman arms are known from other evidence, and in the great legionary fortresses, as well as in the smaller forts and outposts,—established, altered, or abandoned, as the tide of conquest ebbed and flowed across the Rhine,— deposits have been identified as belonging to definite periods, sometimes periods of comparatively short duration. -

Archaeological and Historic Pottery Production Sites Guidelines for Best Practice Summary
Archaeological and Historic Pottery Production Sites Guidelines for Best Practice Summary This document provides practical guidance on how to investigate sites where pottery production has taken place. It describes how to anticipate and locate pottery production sites and the types of evidence that may be found. This document also provides advice on the available methods and strategies for examining, recording and sampling features and finds of various types at each stage of the work. The different techniques for establishing the date of pottery production, and for characterising the products of a site, are given particular emphasis. This document was compiled by Harriet White, Sarah Paynter and Duncan Brown with contributions by Joanne Best, Chris Cumberpatch, David Dawson, Peter Ellis, Jane Evans, Laurence Jones, Oliver Kent, Gareth Perry, The Prince’s Regeneration Trust, Ian Roberts, Kerry Tyler and Ann Woodward. It is one of four Historic England publications concerning industrial processes in the past, the other three being: ■ Science for Historic Industries: Guidelines for the Investigation of 17th- to 19th- Century Industries ■ Archaeological Evidence for Glassworking: Guidelines for Best Practice ■ Archaeometallurgy: Guidelines for Best Practice. First published by Historic England October 2015. All images © Historic England unless otherwise stated. www.historicengland.org.uk/advice/technical-advice/archaeological-science/ Front cover images: clockwise from top left Reconstruction of a 17th-century kiln from Potters A 14th-century -

The Process of Pit Firing
The Process of Pit Firing Introduction My interest in pit fired pottery comes from my desire to strip back the complexities of life and to demonstrate the essential processes involved in the production of pottery. So much of our lives is surrounded by mass production that is epitomised by catalogues of mass produced wares from Asia and these wares can be bought at very cheap prices from homewares shops . These items are functional, well produced but lack character or expression of individuality that would make them objects of art. Early pottery has been found in places such as Jomon, Japan (10,500 BC), but the earliest ceramic objects like the Venus of Dolní Věstonice figurine discovered in the Czech Republic, date back to around 25,000 BC, and pottery vessels from Jiangxi, China date back to 20,000 BC. The earliest pottery probably developed by accident and there are two main theories of development. It may have been from observing how the earth became baked around fire pits or it may have come from the accidental burning of clay-lined baskets. The clay was used to line baskets made of natural fibre in order prevent loss of grains or seeds that were stored in such baskets. Pottery developed in response to needs and so pots had a purpose. This included storing grains or flour, carting, cooking pots, ritual and funeral rites. The rulers of Egypt had their internal organs mummified in canopic jars. The first use of pottery vessels for storing water and food is probably around 9000 or 10,000 BC and so pottery is one of man’s most ancient industries.