The Bajocian (Middle Jurassic): a Key Interval in the Early Mesozoic Phytoplankton Radiation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diversity Partitioning During the Cambrian Radiation
Diversity partitioning during the Cambrian radiation Lin Naa,1 and Wolfgang Kiesslinga,b aGeoZentrum Nordbayern, Paleobiology and Paleoenvironments, Friedrich-Alexander-Universität Erlangen-Nürnberg, 91054 Erlangen, Germany; and bMuseum für Naturkunde, Leibniz Institute for Research on Evolution and Biodiversity at the Humboldt University Berlin, 10115 Berlin, Germany Edited by Douglas H. Erwin, Smithsonian National Museum of Natural History, Washington, DC, and accepted by the Editorial Board March 10, 2015 (received for review January 2, 2015) The fossil record offers unique insights into the environmental and Results geographic partitioning of biodiversity during global diversifica- Raw gamma diversity exhibits a strong increase in the first three tions. We explored biodiversity patterns during the Cambrian Cambrian stages (informally referred to as early Cambrian in this radiation, the most dramatic radiation in Earth history. We as- work) (Fig. 1A). Gamma diversity dropped in Stage 4 and de- sessed how the overall increase in global diversity was partitioned clined further through the rest of the Cambrian. The pattern is between within-community (alpha) and between-community (beta) robust to sampling standardization (Fig. 1B) and insensitive to components and how beta diversity was partitioned among environ- including or excluding the archaeocyath sponges, which are po- ments and geographic regions. Changes in gamma diversity in the tentially oversplit (16). Alpha and beta diversity increased from Cambrian were chiefly driven by changes in beta diversity. The the Fortunian to Stage 3, and fluctuated erratically through the combined trajectories of alpha and beta diversity during the initial following stages (Fig. 2). Our estimate of alpha (and indirectly diversification suggest low competition and high predation within beta) diversity is based on the number of genera in published communities. -

The Middle Jurassic of Western and Northern Europe: Its Subdivisions, Geochronology and Correlations
The Middle Jurassic of western and northern Europe: its subdivisions, geochronology and correlations John H. Callomon The palaeogeographic settings of Denmark and East Greenland during the Middle Jurassic are outlined. They lay in the widespread epicontinental seas that covered much of Europe in the post-Triassic transgression. It was a period of continuing eustatic sea-level rise, with only distant connections to world oceans: to the Pacific, via the narrow Viking Straits between Greenland and Norway and hence the arctic Boreal Sea to the north; and to the subtropical Tethys, via some 1200 km of shelf-seas to the south. The sedimentary history of the region was strongly influenced by two factors: tectonism and climate. Two modes of tectonic movement governed basinal evolution: crustal extension lead- ing to subsidence through rifting, such as in the Viking and Central Grabens of the North Sea; and subcrustal thermal upwelling, leading to domal uplift and the partition of marine basins through emergent physical barriers, as exemplified by the Central North Sea Dome with its associated volcanics. The climatic gradient across the 30º of temperate latitude spanned by the European seas governed biotic diversity and biogeography, finding expression in rock-forming biogenic carbonates that dominate sediments in the south and give way to largely siliciclastic sediments in the north. Geochronology of unrivalled finesse is provided by standard chronostratigraphy based on the biostratigraphy of ammonites. The Middle Jurassic saw the onset of considerable bioprovincial endemisms in these guide-fossils, making it necessary to construct parallel standard zonations for Boreal, Subboreal or NW European and Submediterranean Provinces, of which the NW European zonation provides the primary international standard. -

Natural Communities of Michigan: Classification and Description
Natural Communities of Michigan: Classification and Description Prepared by: Michael A. Kost, Dennis A. Albert, Joshua G. Cohen, Bradford S. Slaughter, Rebecca K. Schillo, Christopher R. Weber, and Kim A. Chapman Michigan Natural Features Inventory P.O. Box 13036 Lansing, MI 48901-3036 For: Michigan Department of Natural Resources Wildlife Division and Forest, Mineral and Fire Management Division September 30, 2007 Report Number 2007-21 Version 1.2 Last Updated: July 9, 2010 Suggested Citation: Kost, M.A., D.A. Albert, J.G. Cohen, B.S. Slaughter, R.K. Schillo, C.R. Weber, and K.A. Chapman. 2007. Natural Communities of Michigan: Classification and Description. Michigan Natural Features Inventory, Report Number 2007-21, Lansing, MI. 314 pp. Copyright 2007 Michigan State University Board of Trustees. Michigan State University Extension programs and materials are open to all without regard to race, color, national origin, gender, religion, age, disability, political beliefs, sexual orientation, marital status or family status. Cover photos: Top left, Dry Sand Prairie at Indian Lake, Newaygo County (M. Kost); top right, Limestone Bedrock Lakeshore, Summer Island, Delta County (J. Cohen); lower left, Muskeg, Luce County (J. Cohen); and lower right, Mesic Northern Forest as a matrix natural community, Porcupine Mountains Wilderness State Park, Ontonagon County (M. Kost). Acknowledgements We thank the Michigan Department of Natural Resources Wildlife Division and Forest, Mineral, and Fire Management Division for funding this effort to classify and describe the natural communities of Michigan. This work relied heavily on data collected by many present and former Michigan Natural Features Inventory (MNFI) field scientists and collaborators, including members of the Michigan Natural Areas Council. -

The Jurassic Pleurotomarioidean Gastropod Laevitomaria and Its Palaeobiogeographical History
The Jurassic pleurotomarioidean gastropod Laevitomaria and its palaeobiogeographical history ROBERTO GATTO, STEFANO MONARI, JÁNOS SZABÓ, and MARIA ALESSANDRA CONTI Gatto, R., Monari, S., Szabó, J., and Conti, M.A. 2015. The Jurassic pleurotomarioidean gastropod Laevitomaria and its palaeobiogeographical history. Acta Palaeontologica Polonica 60 (1): 217–233. The genus Laevitomaria is reviewed and its palaeobiogeographical history is reconstructed based on the re-examination of its type species L. problematica, the study of material stored at the National Natural History Museum of Luxembourg, and an extensive review of the literature. The systematic study allows ascribing to Laevitomaria a number of Jurassic species from the western European region formerly included in other pleurotomariid genera. The following new combi- nations are proposed: Laevitomaria allionta, L. amyntas, L. angulba, L. asurai, L. daityai, L. fasciata, L. gyroplata, L. isarensis, L. joannis, L. repeliniana, L. stoddarti, L. subplatyspira, and L. zonata. The genus, which was once considered as endemic of the central part of the western Tethys, shows an evolutionary and palaeogeographical history consider- ably more complex than previously assumed. It first appeared in the Late Sinemurian in the northern belt of the central western Tethys involved in the Neotethyan rifting, where it experienced a first radiation followed by an abrupt decline of diversity in the Toarcian. Species diversity increased again during Toarcian–Aalenian times in the southernmost part of western European shelf and a major radiation occurred during the Middle Aalenian to Early Bajocian in the northern Paris Basin and southern England. After a latest Bajocian collapse of diversity, Laevitomaria disappeared from both the central part of western Tethys and the European shelf. -

Correlations of the Jurassic Sediments: Infra-Getic Unit
GEOLO[KI ANALI BALKANSKOGA POLUOSTRVA 67 19–33 BEOGRAD, decembar 2006 ANNALES GÉOLOGIQUES DE LA PÉNINSULE BALKANIQUE BELGRADE, December 2006 Tran-sborder (south-east Serbia/west Bulgaria) correlations of the Jurassic sediments: Infra-Getic Unit 1 2 PLATON TCHOUMATCHENCO , DRAGOMAN RABRENOVI] , 3 4 BARBARA RADULOVI] & VLADAN RADULOVI] Abstract. The Infra-Getic Unit is a palaeogeographic unit, predestined by palaeotectonics. From the point of view of geological heritage, it represents a geosites framework. For the purpose of the correlation, the Serbian sections of Lukanja, Bogorodica Monastery, Rosoma~ and Senokos, as well as the Bulgarian sections of Komshtitsa, Gintsi, and Stanyantsi were used. The Jurassic sediments of the Infra-Getic Unit crop out on the southern slops of the Stara Planina Mountain in east Serbia and west Bulgaria. The Lower Jurassic started with continental and continental-marine sediments (clays and sandstones) (Lukanja clastics and Lukanja coal beds in Serbia and the Tuden Formation in Bulgaria) and continue with Lukanja quartz sandstones (Serbia) and the Kostina Formation (Bulgaria). These sediments are covered by Lukanja brachiopod beds and Lukanja limestones (Serbia) and the Romanov Dol, Ravna and Dolni Loukovit Members of the Ozirovo Formation (Bulgaria) pre- dominantly consist of bioclastic limestones. The sedimentations follow with Lukanja belemnites-gryphaea beds (marls and clayey limestones), which in Bulgaria correspond to the Bukorovtsi Member (also marls and clayey limestones) of the Ozirovo Formation. The Middle Jurassic sedimentation started with black shales with Bossitra alpine. These sediments are individualized in Serbia as Senokos aleurolites and clays and in Bulgaria they are known as the Etropole Formation. In Serbia the section continues with sandstones called Vodeni~ki sandstones of Bajocian age, known in Bulgaria as the Dobrogled Member of the Polaten Formation. -

Lower Jurassic to Lower Middle Jurassic Succession at Kopy Sołtysie and Płaczliwa Skała in the Eastern Tatra Mts (Western
Volumina Jurassica, 2013, Xi: 19–58 Lower Jurassic to lower Middle Jurassic succession at Kopy Sołtysie and Płaczliwa Skała in the eastern Tatra Mts (Western Carpathians) of Poland and Slovakia: stratigraphy, facies and ammonites Jolanta IWAŃCZUK1, Andrzej IWANOW1, Andrzej WIERZBOWSKI1 Key words: stratigraphy, Lower to Middle Jurassic, ammonites, microfacies, correlations, Tatra Mts, Western Carpathians. Abstract. The Lower Jurassic and the lower part of the Middle Jurassic deposits corresponding to the Sołtysia Marlstone Formation of the Lower Subtatric (Krížna) nappe in the Kopy Sołtysie mountain range of the High Tatra Mts and the Płaczliwa Skała (= Ždziarska Vidla) mountain of the Belianske Tatra Mts in the eastern part of the Tatra Mts in Poland and Slovakia are described. The work concentrates both on their lithological and facies development as well as their ammonite faunal content and their chronostratigraphy. These are basinal de- posits which show the dominant facies of the fleckenkalk-fleckenmergel type and reveal the succession of several palaeontological microfacies types from the spiculite microfacies (Sinemurian–Lower Pliensbachian, but locally also in the Bajocian), up to the radiolarian microfacies (Upper Pliensbachian and Toarcian, Bajocian–Bathonian), and locally the Bositra (filament) microfacies (Bajocian– Bathonian). In addition, there appear intercalations of detrital deposits – both bioclastic limestones and breccias – formed by downslope transport from elevated areas (junction of the Sinemurian and Pliensbachian, Upper Toarcian, and Bajocian). The uppermost Toarcian – lowermost Bajocian interval is represented by marly-shaly deposits with a marked admixture of siliciclastic material. The deposits are correlated with the coeval deposits of the Lower Subtatric nappe of the western part of the Tatra Mts (the Bobrowiec unit), as well as with the autochthonous-parachthonous Hightatric units, but also with those of the Czorsztyn and Niedzica successions of the Pieniny Klippen Belt, in Poland. -

Appendix 3.Pdf
A Geoconservation perspective on the trace fossil record associated with the end – Ordovician mass extinction and glaciation in the Welsh Basin Item Type Thesis or dissertation Authors Nicholls, Keith H. Citation Nicholls, K. (2019). A Geoconservation perspective on the trace fossil record associated with the end – Ordovician mass extinction and glaciation in the Welsh Basin. (Doctoral dissertation). University of Chester, United Kingdom. Publisher University of Chester Rights Attribution-NonCommercial-NoDerivatives 4.0 International Download date 26/09/2021 02:37:15 Item License http://creativecommons.org/licenses/by-nc-nd/4.0/ Link to Item http://hdl.handle.net/10034/622234 International Chronostratigraphic Chart v2013/01 Erathem / Era System / Period Quaternary Neogene C e n o z o i c Paleogene Cretaceous M e s o z o i c Jurassic M e s o z o i c Jurassic Triassic Permian Carboniferous P a l Devonian e o z o i c P a l Devonian e o z o i c Silurian Ordovician s a n u a F y r Cambrian a n o i t u l o v E s ' i k s w o Ichnogeneric Diversity k p e 0 10 20 30 40 50 60 70 S 1 3 5 7 9 11 13 15 17 19 21 n 23 r e 25 d 27 o 29 M 31 33 35 37 39 T 41 43 i 45 47 m 49 e 51 53 55 57 59 61 63 65 67 69 71 73 75 77 79 81 83 85 87 89 91 93 Number of Ichnogenera (Treatise Part W) Ichnogeneric Diversity 0 10 20 30 40 50 60 70 1 3 5 7 9 11 13 15 17 19 21 n 23 r e 25 d 27 o 29 M 31 33 35 37 39 T 41 43 i 45 47 m 49 e 51 53 55 57 59 61 c i o 63 z 65 o e 67 a l 69 a 71 P 73 75 77 79 81 83 n 85 a i r 87 b 89 m 91 a 93 C Number of Ichnogenera (Treatise Part W) -
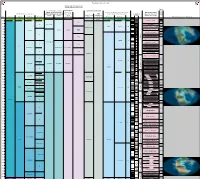
Expanded Jurassic Timescale
TimeScale Creator 2012 chart Russian and Ural regional units Russia Platform regional units Calca Jur-Cret boundary regional Russia Platform East Asian regional units reous stages - British and Boreal Stages (Jur- Australia and New Zealand regional units Marine Macrofossils Nann Standard Chronostratigraphy British regional Boreal regional Cret, Perm- Japan New Zealand Chronostratigraphy Geomagnetic (Mesozoic-Paleozoic) ofossil stages stages Carb & South China (Neogene & Polarity Tethyan Ammonoids s Ma Period Epoch Age/Stage Substage Cambrian) stages Cret) NZ Series NZ Stages Global Reconstructions (R. Blakey) Ryazanian Ryazanian Ryazanian [ no stages M17 CC2 Cretaceous Early Berriasian E Kochian Taitai Um designated ] M18 CC1 145 Berriasella jacobi M19 NJT1 Late M20 7b 146 Lt Portlandian M21 Durangites NJT1 M22 7a 147 Oteke Puaroan Op M22A Micracanthoceras microcanthum NJT1 Penglaizhenian M23 6b 148 Micracanthoceras ponti / Volgian Volgian Middle M24 Burckhardticeras peroni NJT1 Tithonian M24A 6a 149 M24B Semiformiceras fallauxi NJT15 M25 b E 150 M25A Semiformiceras semiforme NJT1 5a Early M26 Semiformiceras darwini 151 lt-Oxf N M-Sequence Hybonoticeras hybonotum 152 Ohauan Ko lt-Oxf R Kimmeridgian Hybonoticeras beckeri 153 m- Lt Late Oxf N Aulacostephanus eudoxus 154 m- NJT14 Late Aspidoceras acanthicum Oxf R Kimmeridgian Kimmeridgian Kimmeridgian Crussoliceras divisum 155 155.431 Card- N Ataxioceras hypselocyclum 156 E Early e-Oxf Sutneria platynota R Idoceras planula Suiningian 157 Cal- Oxf N Epipeltoceras bimammatum 158 lt- Lt Callo -

Calcareous Nannofossil Zonation and Sequence Stratigraphy of the Jurassic System, Onshore Kuwait
GeoArabia, 2015, v. 20, no. 4, p. 125-180 Gulf PetroLink, Bahrain Calcareous nannofossil zonation and sequence stratigraphy of the Jurassic System, onshore Kuwait Adi P. Kadar, Thomas De Keyser, Nilotpaul Neog and Khalaf A. Karam (with contributions from Yves-Michel Le Nindre and Roger B. Davies) ABSTRACT This paper presents the calcareous nannofossil zonation of the Middle and Upper Jurassic of onshore Kuwait and formalizes current stratigraphic nomenclature. It also interprets the positions of the Jurassic Arabian Plate maximum flooding surfaces (MFS J10 to J110 of Sharland et al., 2001) and sequence boundaries in Kuwait, and correlates them to those in central Saudi Arabia outcrops. This study integrates data from about 400 core samples from 11 wells representing a nearly complete Middle to Upper Jurassic stratigraphic succession. Forty-two nannofossil species were identified using optical microscope techniques. The assemblage contains Tethyan nannofossil markers, which allow application of the Jurassic Tethyan nannofossil biozones. Six zones and five subzones, ranging in age from Middle Aalenian to Kimmeridgian, are established using first and last occurrence events of diagnostic calcareous nannofossil species. A chronostratigraphy of the studied formations is presented, using the revised formal stratigraphic nomenclature. The Marrat Formation is barren of nannofossils. Based on previous studies it is dated as Late Sinemurian–Early Aalenian and contains Middle Toarcian MFS J10. The overlying Dhruma Formation is Middle or Late Aalenian (Zone NJT 8c) or older, to Late Bajocian (Subzone NJT 10a), and contains Lower Bajocian MFS J20. The overlying Sargelu Formation consists of the Late Bajocian (Subzone NJT 10b) Sargelu-Dhruma Transition, and mostly barren Sargelu Limestone in which we place Lower Bathonian MFS J30 near its base. -
Letter to the Editor
GeoArabia, Vol. 10, No. 3, 2005 Gulf PetroLink, Bahrain LETTER TO THE EDITOR from Ghaida Al-Sahlan ([email protected]), Kuwait Oil Company, Ahmadi, Kuwait n the recent GeoArabia, Haq and Al-Qahtani (2005) updated the chronostratigraphic Arabian Plate Iframework of Sharland et al. (2001). These studies cite the paper by Yousif and Nouman (1997) to represent the Jurassic type section of Kuwait. Yousif and Nouman published the composite log for the Minagish-27 well (see Figure on page 194) and depicted the Jurassic formations and stages, side-by- side, but only in a generalized manner. In order to refine the ages for this section, I would like to share some preliminary unpublished biostratigraphic and Sr isotope data (see Table and Notes) from analyses by Varol Research (1997 unpublished report), ExxonMobil (1998 unpublished report) and Fugro-Robertson (2004 unpublished report). To convert Sr ages (Ma) to biostages, or biostages to ages, I have used the Geological Time Scale (GTS) 2004 (Gradstein et al., 2004). I thank G.W. Hughes, A. Lomando, M. Miller and O. Varol for their comments. Unit or Boundary Age and Stage Gradstein et al. (2004) Makhul (Offshore) Tithonian-Berriasian (Bio) Base Makhul (N. Kuwait) No younger than Tithonian (Bio) greater than 145.5 + 4.0 Top Hith (W. Kuwait) 150.0 (Sr) = c. Tithonian/Kimmeridgian ? 150.8 + 4.0 Upper Najmah (S. Kuwait) 155.0 (Sr) = c. Kimmeridgian/Oxfordian 155.7 + 4.0 Najmah (N. Kuwait) No older than Oxfordian (Bio) less than 161.2 + 4.0 Lower Najmah Shale (N. Kuwait) middle and late Bathonian (Bio) 166.7 to 164.7 + 4.0 Top Sargelu (S. -

The Late Jurassic Tithonian, a Greenhouse Phase in the Middle Jurassic–Early Cretaceous ‘Cool’ Mode: Evidence from the Cyclic Adriatic Platform, Croatia
Sedimentology (2007) 54, 317–337 doi: 10.1111/j.1365-3091.2006.00837.x The Late Jurassic Tithonian, a greenhouse phase in the Middle Jurassic–Early Cretaceous ‘cool’ mode: evidence from the cyclic Adriatic Platform, Croatia ANTUN HUSINEC* and J. FRED READ *Croatian Geological Survey, Sachsova 2, HR-10000 Zagreb, Croatia Department of Geosciences, Virginia Tech, 4044 Derring Hall, Blacksburg, VA 24061, USA (E-mail: [email protected]) ABSTRACT Well-exposed Mesozoic sections of the Bahama-like Adriatic Platform along the Dalmatian coast (southern Croatia) reveal the detailed stacking patterns of cyclic facies within the rapidly subsiding Late Jurassic (Tithonian) shallow platform-interior (over 750 m thick, ca 5–6 Myr duration). Facies within parasequences include dasyclad-oncoid mudstone-wackestone-floatstone and skeletal-peloid wackestone-packstone (shallow lagoon), intraclast-peloid packstone and grainstone (shoal), radial-ooid grainstone (hypersaline shallow subtidal/intertidal shoals and ponds), lime mudstone (restricted lagoon), fenestral carbonates and microbial laminites (tidal flat). Parasequences in the overall transgressive Lower Tithonian sections are 1– 4Æ5 m thick, and dominated by subtidal facies, some of which are capped by very shallow-water grainstone-packstone or restricted lime mudstone; laminated tidal caps become common only towards the interior of the platform. Parasequences in the regressive Upper Tithonian are dominated by peritidal facies with distinctive basal oolite units and well-developed laminate caps. Maximum water depths of facies within parasequences (estimated from stratigraphic distance of the facies to the base of the tidal flat units capping parasequences) were generally <4 m, and facies show strongly overlapping depth ranges suggesting facies mosaics. Parasequences were formed by precessional (20 kyr) orbital forcing and form parasequence sets of 100 and 400 kyr eccentricity bundles. -

Systematics, Endemism and Phylogeny of Indian Proplanulitins (Ammonoidea) from the Bathonian–Callovian of Kutch, Western India
Swiss J Palaeontol (2016) 135:23–56 DOI 10.1007/s13358-015-0101-2 Systematics, endemism and phylogeny of Indian proplanulitins (Ammonoidea) from the Bathonian–Callovian of Kutch, western India 1 1 Rakhi Dutta • Subhendu Bardhan Received: 25 February 2015 / Accepted: 28 August 2015 / Published online: 8 December 2015 Ó Akademie der Naturwissenschaften Schweiz (SCNAT) 2015 Abstract Spath (1931) described five genera namely Si- Bathonian of Kutch (Roy et al. 2007). Procerites was a vajiceras Spath, Obtusicostites Buckman, Hubertoceras European genus and perhaps gave rise to Proplanulites in Spath, Kinkeliniceras Buckman and Cutchisphinctes Spath the Boreal Europe. We here envisaged that Sivajiceratinae from the Upper Bathonian and entire Callovian of Kutch, and the European Proplanulitinae show evolutionary con- western India and included 33 species within them. He vergence of some characters since they evolved from a grouped these genera within the subfamily Proplanulitinae common ancestral stock. of the Boreal Province. But however the palaeobiogeo- graphic distributions of the Kutch forms suggest that they Keywords Sivajiceratinae Á New subfamily Á Kutch Á were restricted within the Indo-Madagascan Province. Middle Jurassic Á Palaeobiogeography Á Evolution Callomon (1993) expressed doubts about the phylogenetic affinity of Kutch genera and inferred that proplanulitins of Kutch were unrelated to Boreal Proplanulites and consti- Introduction tute an endemic lineage. In the present study, we made a thorough systematic revision of proplanulitin taxa of Kutch The Bathonian of Kutch, western India, witnessed sudden in the light of intraspecific variability and sexual dimor- migration of many ammonite genera from different pro- phism. Our study reveals that Spath’s (1931) work suffered vinces as soon as the Kutch Basin opened up (Roy et al.