Vascularisation of Pluripotent Stem Cell–Derived Myocardium: Biomechanical Insights for Physiological Relevance in Cardiac Tissue Engineering
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
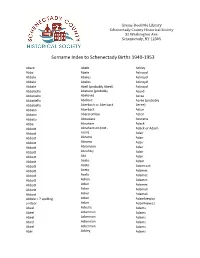
Surname Index to Schenectady Births 1940-1953
Grems-Doolittle Library Schenectady County Historical Society 32 Washington Ave. Schenectady, NY 12305 Surname Index to Schenectady Births 1940-1953 Abare Abele Ackley Abba Abele Ackroyd Abbale Abeles Ackroyd Abbale Abeles Ackroyd Abbale Abell (probably Abeel) Ackroyd Abbatiello Abelone (probably Acord Abbatiello Abelove) Acree Abbatiello Abelove Acree (probably Abbatiello Aberbach or Aberback Aeree) Abbato Aberback Acton Abbato Abercrombie Acton Abbato Aboudara Acucena Abbe Abraham Adack Abbott Abrahamson (not - Adack or Adach Abbott nson) Adair Abbott Abrams Adair Abbott Abrams Adair Abbott Abramson Adair Abbott Abrofsky Adair Abbott Abt Adair Abbott Aceto Adam Abbott Aceto Adamczak Abbott Aceto Adamec Abbott Aceto Adamec Abbott Acken Adamec Abbott Acker Adamec Abbott Acker Adamek Abbott Acker Adamek Abbzle = ? spelling Acker Adamkiewicz unclear Acker Adamkiewicz Abeel Ackerle Adams Abeel Ackerman Adams Abeel Ackerman Adams Abeel Ackerman Adams Abeel Ackerman Adams Abel Ackley Adams Grems-Doolittle Library Schenectady County Historical Society 32 Washington Ave. Schenectady, NY 12305 Surname Index to Schenectady Births 1940-1953 Adams Adamson Ahl Adams Adanti Ahles Adams Addis Ahman Adams Ademec or Adamec Ahnert Adams Adinolfi Ahren Adams Adinolfi Ahren Adams Adinolfi Ahrendtsen Adams Adinolfi Ahrendtsen Adams Adkins Ahrens Adams Adkins Ahrens Adams Adriance Ahrens Adams Adsit Aiken Adams Aeree Aiken Adams Aernecke Ailes = ? Adams Agans Ainsworth Adams Agans Aker (or Aeher = ?) Adams Aganz (Agans ?) Akers Adams Agare or Abare = ? Akerson Adams Agat Akin Adams Agat Akins Adams Agen Akins Adams Aggen Akland Adams Aggen Albanese Adams Aggen Alberding Adams Aggen Albert Adams Agnew Albert Adams Agnew Albert or Alberti Adams Agnew Alberti Adams Agostara Alberti Adams Agostara (not Agostra) Alberts Adamski Agree Albig Adamski Ahave ? = totally Albig Adamson unclear Albohm Adamson Ahern Albohm Adamson Ahl Albohm (not Albolm) Adamson Ahl Albrezzi Grems-Doolittle Library Schenectady County Historical Society 32 Washington Ave. -

Surname Given Age Date Page Maiden Note Abildua Frank 91 22-Dec D-1 Ables Cary James 14 13-Apr D-1 Full Name Abrahamson Ethel 91 9-Mar D-2 Newman Abramson Rose H
Surname Given Age Date Page Maiden Note Abildua Frank 91 22-Dec D-1 Ables Cary James 14 13-Apr D-1 Full name Abrahamson Ethel 91 9-Mar D-2 Newman Abramson Rose H. 81 2-Feb D-2 Accordini Mary G. 70 20-Dec A-13 Ackerman Frances L. 4-May C-5 Adams Eva 83 3-Apr D-2 Stewart Adams Michael J. 76 28-Jan D-1 Adell Alfred S. 48 5-May B-4 Adoba William J., Sr. 64 10-Jan B-6 Agnew W. Lynn 85 12-Sep B-6 Albaugh James 81 9-Oct E-1 Albee Daniel 62 27-Jul D-1 Albert Henrietta 79 13-Apr D-1 Albright Ralph G. 80 13-Apr D-1 Aldrin Myrtle A. 76 15-Jan D-6 Alexander Daniel L. 37 13-Jun C-5 Alexander W. (Rev.) 69 6-Jan D-2 Alexich Jacov "Jack" 60 26-Dec D-1 Allande Arthur 80 13-Oct D-3 Allande Augustina 79 2-May C-2 Allen James P., Sr. 49 30-Oct D-1 Allen Ernest L. 48 6-Sep B-5 Veteran of the Korean conflict Allen Lizzie 3-Jan A-11 Allen William M. 75 21-Mar C-2 Allen Aryln J. 60 5-Jun F-7 Allen Annie 101 4-Feb D-1 Aller Edward D. 81 11-Jan D-2 Almanza Maria 81 13-Feb D-1 Aloia Frank, Sr. 90 9-Feb C-2 Alongi Samuel 70 5-Jan C-5 Veteran of World War II Alpert Samual 97 15-Dec C-7 Alsdorf Rose Evelyn 52 30-Sep C-3 Full name Alsip Yvetta E. -

Activation De La Phosphodiestérase De Type 2 Pour Traiter L'insuffisance Cardiaque
NNT : 2016SACL336 THESE DE DOCTORAT DE L’UNIVERSITE PARIS-SACLAY PREPAREE A UNIVERSITE PARIS SUD ECOLE DOCTORALE N° 569 ITFA Innovation thérapeutique: du fondamental à l’appliqué Spécialité de doctorat: Physiologie, physiopathologie Par Marta Lindner Activation de la phosphodiestérase de type 2 pour traiter l'insuffisance cardiaque Thèse présentée et soutenue à Châtenay-Malabry, le 12 Octobre 2016 Composition du Jury : M. Christian POÜS Professeur, UPSud Président Mme Claire LUGNIER DR Emérite CNRS Rapporteur Mme Catherine PAVOINE CR INSERM Rapporteur Mme Liliana CASTRO Maître de Conférence, UPMC Examinateur M. Ali EL-ARMOUCHE Professeur, Univ. Dresden Examinateur M. Grégoire VANDECASTEELE DR INSERM Examinateur M. Rodolphe FISCHMEISTER DR INSERM Directeur de thèse Table of contents Sommaire Introduction ______________________________________________________________ 13 I. The heart _______________________________________________________________ 14 I.1 Anatomy of the heart ________________________________________________________ 14 I.2 Cardiac excitation-contraction coupling __________________________________________ 15 I.2.1 Structures involved in cardiac ECC _____________________________________________________ 15 I.2.1.1 Sarcomere and T-tubules _______________________________________________________ 15 I.2.1.2 Sarcoplasmic reticulum ________________________________________________________ 17 I.2.1.3 Myofilaments ________________________________________________________________ 17 I.2.1.4 Mitochondria ________________________________________________________________ -
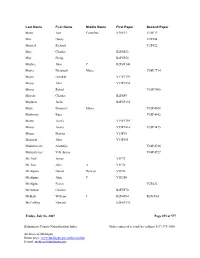
Kalamazoo County Naturalization Index Order Copies of Records by Calling (517) 373-1408
Last Name First Name Middle Name First Paper Second Paper Maus Joos Cornelius V30P33 V30P33 Max Henry V2P204 Maxted Richard V2P422 May Charles B2F6P23 May Philip B2F6P55 Maybee John C B2F6P140 Mayer Elizabeth Marie V58P3714 Mayer Hendrik V17P3359 Mayer John V17P3234 Mayer Robert V51P3086 Mayers Charles B2F6P9 Mayhew Jacob B2F6P135 Mayr Emanuele Maria V62P4308 Mazkrists Ruta V65P4942 Mazur Josefa V19P3789 Mazur Josefa V55P3416 V55P3415 Mazur Marcin V15P18 Mazurak John V13P291 Mazurkevics Anatolijs V64P4726 Mazurkevics Vilhelmina V64P4727 Mc Neil James V5P72 Mc Rae John A V5P76 McAlpine Daniel Duncan V3P26 McAlpine John C V3P250 McAlpine Peeter V2P426 McArthur Charles B2F5P70 McBeth William L B2F6P84 B2F6P84 McCaffrey Edward B2F6P136 Friday, July 06, 2007 Page 299 of 577 Kalamazoo County Naturalization Index Order copies of records by calling (517) 373-1408 Archives of Michigan Home page: www.michigan.gov/archivesofmi E-mail: [email protected] Last Name First Name Middle Name First Paper Second Paper McCall Hannah V16P3084 McCallum Duncan Clyde V10P370 McCallum Duncan Clyke V23P212 V23P212 McCally Geoffrey Thomas V52P3165 McCamby Alex B2F6P1 McCamly Daniel B2F6P5 McCandless Frances Georgina Forsythe V61P4262 McCandless Hugh Milligan V20P4331 McCandry Daniel B2F6P5 McClelland George Charles V23P101 McClure Mary Abernethy V10P389 McClurky Charles B2F6P67 McCormick George W V5P33 McCormick Olga V48P2744 McCray Robert B2F6P157 McCudden John B2F6P150 McCue Hugh B2F5P56 McCue John B2F6P76 McCue Peter V62P4379 McCugan Vincent Murray V19P3955 -

2015 Annual Report
t r o p e R l a u n An 2015 2015 Board of Directors Officers Joel Huotari President Emily Hartzog 1st Vice President Kurt Broski 2nd Vice President Dayton B. Smith III Treasurer Colleen Anderson Secretary Sam Castree Past President Sarah Wolf Executive Director Directors Growing…Volunteers! Brian Blakemore Discovery Center has grown from our foundational roots which Nathan Boelkins were started by volunteers. Our community, whether small or Karen Brown Lesly Couper tall, are a vital resource for helping us advance our mission! Tony Foti James Horton total volunteers Ehren Jarrett 482 Scott Kaiser active Youth Experiencing Science (YES) Michelle Klaman volunteers (ages 13-17) Kelly Lattimer 150 Carol Mittel Tony Moczynski individual adults Lindsay Moore 40 Denise Noe Board of Director members contributed Lisa A. Normoyle over hours of their expertise James Pirages 24 670 Jeffery Weatherall business and school groups Gordon Wright 26 Auxiliary volunteer hours contributed to the museum Committee Members Deb Boland 5500+ Sarah Butenhoff Audra Capriola Christina Elbers Growing...Recognition! Nina Jacobson Discovery Center was awarded a TripAdvisor® Certificate Laura Kenrick of Excellence and was recognized as a Top Family Julia Kindler Attraction Worth Traveling For in the Andrea Mandala U.S. by Flipkey/TripAdvisor. Bill Miller Jennie Polizzotto Rockford Register Star annual What Rocks contest Catherine Povalitis Best Child-friendly Place Liz Wood B103 Radio Station Carol Wright Best Place to take the Kids in Rockford 2 DISCOVERY CENTER MUSEUM ANNUAL REPORT 2015 Growing…Exploration! Power of Electricity This new exhibit provides high quality 21st century learning experiences in STEAM, inspiring the next generation through interaction and energy stimulation. -

Obituary Index
Yamhill County Obituary Index Surnames:M-Z Obituaries contained in the files at the Yamhill County Historical Society Research Library in Lafayette, Oregon 1850s through October 2019 Yamhill County Historical Society 657 Market Street Lafayette, Oregon 97127 www.yamhillcountyhistory.org Obituary clippings are pasted to note cards and filed alphabetically. Copies can be obtained by contacting us at by phone or email, or in person during our open hours. Current contact information can be found at www.yamhillcountyhistory.org A duplication fee will be incurred for copies made. Obituaries have been gathered from current and discontinued newspapers Local Newspapers Include: • News Register (McMinnville) • Newberg Graphic • Sheridan Sun Regional Newspapers • Oregonian (Portland) • Statesman Journal (Salem) Maahs, Faythe Dec 25, 1999 Maahs, Leonard H Apr 12, 2003 Maas, Agnes “Peggy” May 12, 1975 Maas, Catharina Wilhelmina Aug 22, 2010 Maas, Edna E Jan 20, 1981 Maas, Jacob B Apr 2, 1984 Maas, Jerry B Aug 27, 2005 Maas, Petrus G “Peter” Jul 24, 2002 Mabal, Linnie Bewley Feb 16, 1930 Mabee, Donald F Nov 14, 1996 Mabee, Matilda Mar 8, 1937 Mabee, Susan Bernice Apr 13, 2017 Maben, Richard Lee Mar 24, 2018 Maben, Robert “Bob” May 20, 2019 Mabry, Doris A Nov 17, 2005 Mabry, Jack Roger Jul 26, 2012 Mabry, Laura Belle Mar 23, 2018 Mabry, Mildred R Jan 2, 2012 MacArthur, Donald F Dec 8, 2009 Macauley, Donna Lynn Nov 12, 1989 Macauley, John G Nov 6, 1974 Macauley, Margaret Jul 13, 2010 Macauley, Murray “Neil” Nov 12, 2008 MacBeth, Gordon Dec 10, 2014 MacBeth, -

Names from City Directories, 1876-1889
City Directory Death Dates, Removed To, and Marriages 1876 - 1889 Dir. Year Surname Given Month Day Year Age Removed: 1884 Aab Thomas A. to Poughkeepsie 1888 Aaron Christopher B. to Erie, Pa. 1880 Abberger Simon 6 1879 1883 Abbey Ashahel M. to Kansas City, Mo. 1886 Abbey Charlotte A. to Kankakee, Ill. 1884 Abbott Adoniram J. to Geneseo 1887 Abbott Benjamin V. to New York City 1887 Abbott Charles H. to Chicago, Ill. 1889 Abbott Charles M. from city 1887 Abbott Fred. L. to Syracuse 1884 Abbott John B. to Geneseo 1883 Abbott Luman 11 9 1882 1882 Abbott S. Augustus to Eau Claire, Wis. 1887 Abbott Walter S. to Gananoque, Can. 1879 Abel Bernard to Fowlerville 1876 Abel Lester from city 1882 Abel Sarah B. to Greece 1884 Abeles Henry from city 1884 Abell Joseph to San Francisco, Cal. 1885 Abels Joseph 12 21 1884 39 1880 Abercrombie Archibald from city 1879 Abey Harry T. to Schroon Lake 1887 Abner Edward to Brooklyn 1876 Abrams George from city 1888 Absom William to Cincinnati, Oh. 1885 Acer Murray from city 1884 Achilles Charles B. to Palatka, Fla. 1879 Achilles Charles P. 9 20 1878 to Tacoma, 1888 Achilles Henry L., Jr. Washington Territory 1881 Acker Daniel F. from city 1888 Acker Jacob to Bellville, Canada 1884 Acker Jacob B. to Rome 1886 Acker Jacob B. to Ovid, N. Y. 1876 Acker John H. to Troy 1885 Ackerman Frank 12 31 1884 23 1885 Ackerman Leslie W. to Ogdensburg 1885 Ackerman Philip M. to Gates 1883 Ackerman Philip S. 11 15 1882 1877 Ackes Jacob to California 1889 Adair Peter to St. -

Committee on Faculty, Staff and Administration Jun 7, 2021 4:00 PM - 7:00 PM EDT
Committee on Faculty, Staff and Administration Jun 7, 2021 4:00 PM - 7:00 PM EDT Table of Contents I. ACTION ITEMS: A. Approval of the Minutes of the Meeting of May 3, 2021...........................................2 B. POLICY CALENDAR: 1. RESOLUTION TO Approve the Committee on Faculty, Staff and Administration Report..............................................................................................7 2. RESOLUTION TO Amend the Governance Plan of Hostos Community College at The City University of New York.........................................................44 3. RESOLUTION TO Appoint Linda Essig as Provost and Senior Vice President of Academic Affairs at Baruch College at The City University of New York.....76 4. RESOLUTION TO Appoint Teresa Bandosz as Distinguished Professor at The City College of The City University of New York..........................................92 5. RESOLUTION TO Appoint Cecilia Maria Gonzalez-McHugh as Distinguished Professor at Queens College of The City University of New York...................167 6. RESOLUTION TO Award Sofya Aptekar with Early Tenure at The School of Labor of The City University of New York with an Application of Bylaw 6.2.c(2)...................................................................................................................223 7. RESOLUTION TO Award Mary Theresa Kiely with Early Tenure at Queens College of The City University of New York with an Application of Bylaw 6.2.c(2)...................................................................................................................238 -

MAAS - 625B MACARD, Michelle Madeleine (Mrs
MAAS - 625B MACARD, Michelle Madeleine (Mrs. Charles CADIEU dit COURVILLE, I) - 286B-16A MACIER - 504A MACLIN, Marguarite (Mrs. Jean J. CHIQUOT/SICOT) - 266B-2M MACLIN, Nicolas (Susanne LAROSE) - 266B-3M MADSEN, Holger (Emma JENSEN) - 373B-Fg-4a MADSEN, Jens (Kirsten M. CHRISTENSDATTER) - 230B-3 MAGGIO, Camelia (Mrs. John H. SCHAUMANN) - 405B-1 MAGGIO, Rosario (Sarah I. HANDLEY) - 405B-1 MAGNER - 532A MAGNON, Jeanne (Mrs. Francois BIVILLE, I) - 377A-55 MAGNON, Jeanne (Mrs. Francois BIVILLE dit LePICARD, I) - 235-C84 MAGNON/MAGNIER/MIGNIER, M. (Mrs. Nicolas BELANGER, III) - 286B-16 MAGRUDER, Elizabeth Ann (Mrs. Hugh LANCASTER) - 359B MAHANNAH: Abraham & Levi/Sylvester (ch of Nicholas & M.Lavina MAHANNAH)-585A-Fg10 MAHANNAH, Carlisle/Steward (ch of Nicholas & M.Lavina MAHANNAH) - 585A-Fg-10 MAHANNAH, Evelyn A. - 585A-1,Fg7/8/9 MAHANNAH, Harry (Lois L. TAYLOR) - 585A-Fg-9 MAHANNAH, Harvey (#3-Margaret A. MOORE) - 585A-Fg-10 MAHANNAH, Harvey (#2-Pluma M. COWDERY) - 585A-Fg-10 MAHANNAH, Harvey (#1-Abbie RALPH) - 585A-Fg-10 MAHANNAH, John (Linnie P. FOSTER) - 585A-Fg9 MAHANNAH: Martha, Jerome, & Eleanor (ch of Nicholas & M/Lavina ) - 585A-Fg-10 MAHANNAH, Mary Ann (Mrs. Joseph DAVIS) - 585A-Fg-10 MAHANNAH/MOHANNAH, Evelyn/Eva A. (#2-Mrs. Thomas BINGHAM) - 585A-Fg-7,9 MAHANNAH/MOHANNAH, Evelyn/Eva A. (#1-Mrs. John F. BOLES) - 585A-1,Fg-7,8,9 MAHANNAH, Naomi (Mrs. John J. QUINN) - 585A-Fg-9 MAHANNAH, Nicholas J. (Mary/Lavina DAVIS) - 585A-Fg-9,10 MAHANNAH, Nicholas (Eliza >Unknown=) - 585A-Fg-10 MAHANNAH, Rebecca (Mrs. James CHRISTY) - 585A-Fg-10 MAHANNAH, Samuel W. (Georgiana SOUTHERN) - 585A-Fg-9 MAHANNAH, Samuel (#3-M. -

Master Question List for COVID-19 (Caused by SARS-Cov-2) Monthly Report 13 August 2021
DHS SCIENCE AND TECHNOLOGY Master Question List for COVID-19 (caused by SARS-CoV-2) Monthly Report 13 August 2021 For comments or questions related to the contents of this document, please contact the DHS S&T Hazard Awareness & Characterization Technology Center at [email protected]. DHS Science and Technology Directorate | MOBILIZING INNOVATION FOR A SECURE WORLD CLEARED FOR PUBLIC RELEASE REQUIRED INFORMATION FOR EFFECTIVE INFECTIOUS DISEASE OUTBREAK RESPONSE SARS-CoV-2 (COVID-19) Updated 8/13/2021 FOREWORD The Department of Homeland Security (DHS) is paying close attention to the evolving Coronavirus Infectious Disease (COVID-19) situation in order to protect our nation. DHS is working very closely with the Centers for Disease Control and Prevention (CDC), other federal agencies, and public health officials to implement public health control measures related to travelers and materials crossing our borders from the affected regions. Based on the response to a similar product generated in 2014 in response to the Ebolavirus outbreak in West Africa, the DHS Science and Technology Directorate (DHS S&T) developed the following “master question list” that quickly summarizes what is known, what additional information is needed, and who may be working to address such fundamental questions as, “What is the infectious dose?” and “How long does the virus persist in the environment?” The Master Question List (MQL) is intended to quickly present the current state of available information to government decision makers in the operational response to COVID-19 and allow structured and scientifically guided discussions across the federal government without burdening them with the need to review scientific reports, and to prevent duplication of efforts by highlighting and coordinating research. -

Surname Given Age Date Page Maiden Note Year Abel Ronald G. 78 February 21 A-7 2017 Abraham Leonard F. 76 October 25 A-10 2017 A
Surname Given Age Date Page Maiden Note Year Abel Ronald G. 78 February 21 A-7 2017 Abraham Leonard F. 76 October 25 A-10 2017 Abramson Cathy 61 August 26 A-6 Powers 2017 Absber Leroy Newt "Roy" 89 January 4 A-5 Picture Included 2017 Sobuh/Abu Abu + Amera 17 October 26 A-8 Picture Included 2017 Hakmeh Ackerman Elizabeth M. 75 October 18 A-12 Star of David Included 2017 Acquafredda Lorraine H. 85 March 7 A-5 Dopinak 2017 Adamczyk Robert L., Sr. 58 January 5 A-6 2017 Adams Bill 94 June 7 A-10 2017 Caroline Reed Thrun, Adams 87 July 6 A-8 Bess 2017 "Chuggie" Adams Charles Wayne "Chuck" 65 April 27 A-8 Picture Included 2017 Adams Joseph L. 83 November 1 A-8 2017 Adams Loretta V., "Penny" 90 August 5 A-6 Penn 2017 Adams Nellie Jean 84 July 2 A-10 Millender Picture Included 2017 Adams Shirley 85 January 19 A-10 Hicks 2017 Adams Susan Mary 60 February 23 A-7 Thanholt 2017 Adams Virginia L. 91 September 29 A-8 Bicknell 2017 Adamski Paul 70 June 3 A-6 2017 Addison Georgia M. 94 January 26 A-9 Olson 2017 Addison James Edward 57 May 3 A-10 Picture Included 2017 Adduci Donald R. 58 April 30 A-10 2017 Adkins Boice Neal 67 February 3 A-6 2017 Adler Lorraine J. 84 June 27 A-6 Idzior Picture Included 2017 Aguilera Arturo A. 85 February 13 A-8 2017 Aguilera Betty J. 68 March 1 A-8 2017 Frances "Mumu," Aguirre 77 December 28 A-11 Hernandez 2017 "Chacha" Ailes Rilla Lilian 89 February 12 A-12 2017 Albanese Richard 88 August 23 A-9 Flag and Picture Included 2017 Albee Patricia A., "Patti" 58 December 28 A-11 Kieltyka 2017 Albee Patricia, A, "Patti" 58 November 27 A-8 Kieltyka 2017 Albert John Richard 74 September 28 A-7 Picture Included 2017 Albertson Ivan Glenn 71 April 25 A-8 2017 Aldridge Bobby V. -

AMEE 2008 Final Programme
2008 Prague, Czech Republic 30 August to 3 September 2008 PROGRAMME Association for Medical Education in Europe (AMEE) with the endorsement of: Tay Park House, 484 Perth Road, Dundee DD2 1LR, UK Tel: +44 (0)1382 381953 Fax: +44 (0)1382 381987 Email: [email protected] Scottish Charity No. SC 031618 www.amee.org 2009 29 August – 2 September Palacio de Ferias y Congresos de Málaga Málaga, Spain Pre-conference workshops Saturday 29 & Sunday 30 August Main Conference Sessions Monday 31 August to Wednesday 2 September In collaboration with XIX SEDEM Meeting Sociedad Española de Educación Médica Suggestions for topics, symposia, plenary speakers & pre-conference workshops are invited by 30 September 2008 Call for papers: December 2008 Abstract deadlines: Research papers by 21 January 2009 Other papers by 13 March 2009 Association for Medical Education in Europe Tay Park House, 484 Perth Road, Dundee DD2 1LR, Scotland, UK Tel: +44 (0)1382 381953 Fax: +44 (0)1382 381987 email: [email protected] http://www.amee.org Scottish Charity No. SC031618 Pre-Conference Workshops and Courses Saturday 30 August Date Club Club Club Club Club Club Room Room Room Room Terrace Dressing Dressing Dressing Room Room Meeting A B C D E H 2.1 2.2 2.3 2.4 2 Rm 220 Rm 221 Rm 222 1.1 3.1 Hall II COURSE COURSE PCW5 PCW3 COURSE COURSE PCW1 PCW6 PCW4 COURSE PCW2 PCW7 SATURDAY 0900-1700 0900-1700 0900-1700 0900-1700 30 AUGUST morning 0915-1215 CME: Part 1 Role-play ESME Course FAME Course FAME Challenges of RESME Course Tuning Project Tuning ESTEME Course clinical teaching