Benzene from Wikipedia, the Free Encyclopedia See Also: Benzole
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pyrylium Salt Chemistry Emerging As a Powerful Approach for the Cite This: Chem
Chemical Science View Article Online PERSPECTIVE View Journal | View Issue Over one century after discovery: pyrylium salt chemistry emerging as a powerful approach for the Cite this: Chem. Sci., 2020, 11, 12249 All publication charges for this article construction of complex macrocycles and metallo- have been paid for by the Royal Society of Chemistry supramolecules Yiming Li, ab Heng Wanga and Xiaopeng Li *a Over one century after its discovery, pyrylium salt chemistry has been extensively applied in preparing light emitters, photocatalysts, and sensitizers. In most of these studies, pyrylium salts acted as versatile precursors for the preparation of small molecules (such as furan, pyridines, phosphines, pyridinium salts, thiopyryliums and betaine dyes) and poly(pyridinium salt)s. In recent decades, pyrylium salt chemistry has emerged as a powerful approach for constructing complex macrocycles and metallo-supramolecules. In this perspective, we attempt to summarize the representative efforts of synthesizing and self-assembling Received 20th August 2020 large, complex architectures using pyrylium salt chemistry. We believe that this perspective not only Creative Commons Attribution-NonCommercial 3.0 Unported Licence. Accepted 13th October 2020 highlights the recent achievements in pyrylium salt chemistry, but also inspires us to revisit this chemistry DOI: 10.1039/d0sc04585c to design and construct macrocycles and metallo-supramolecules with increasing complexity and rsc.li/chemical-science desired function. 1. Introduction pyrylium salt with perchlorate as the counterion was reported in 1911 by Baeyer;7 however, since the discovery of pyrylium salts, Pyrylium salts are a type of six-membered cationic heterocycles such salts have been underappreciated for about a half-century. -

AIKO ADAMSON Properties of Amine-Boranes and Phosphorus
DISSERTATIONES CHIMICAE AIKO ADAMSON AIKO UNIVERSITATIS TARTUENSIS 138 Properties of amine-boranes and phosphorus analogues in the gas phase Properties AIKO ADAMSON Properties of amine-boranes and phosphorus analogues in the gas phase Tartu 2014 ISSN 1406-0299 ISBN 978-9949-32-627-3 DISSERTATIONES CHIMICAE UNIVERSITATIS TARTUENSIS 138 DISSERTATIONES CHIMICAE UNIVERSITATIS TARTUENSIS 138 AIKO ADAMSON Properties of amine-boranes and phosphorus analogues in the gas phase Institute of Chemistry, Faculty of Science and Technology, University of Tartu. Dissertation is accepted for the commencement of the Degree of Doctor philo- sophiae in Chemistry on June 18, 2014 by the Doctoral Committee of the Institute of Chemistry, University of Tartu. Supervisor: prof. Peeter Burk (PhD, chemistry), Institute of Chemistry, University of Tartu, Estonia Opponent: prof. Holger Bettinger (PhD), University Tuebingen, Germany Commencement: August 22, 2014 at 10:00, Ravila 14a, room 1021 This work has been partially supported by Graduate School „Functional materials and technologies” receiving funding from the European Social Fund under project 1.2.0401.09-0079 in University of Tartu, Estonia. Publication of this dissertation is granted by University of Tartu ISSN 1406-0299 ISBN 978-9949-32-627-3 (print) ISBN 978-9949-32-628-0 (pdf) Copyright: Aiko Adamson, 2014 University of Tartu Press www.tyk.ee CONTENTS LIST OF ORIGINAL PUBLICATIONS ....................................................... 6 1. INTRODUCTION .................................................................................... -

Direct, Catalytic, Intermolecular Anti
DIRECT, CATALYTIC, INTERMOLECULAR ANTI-MARKOVNIKOV HYDROACETOXYLATIONS OF ALKENES ENABLED VIA PHOTOREDOX CATALYSIS, AND INVESTIGATIONS INTO THE MECHANISM OF THE POLYMERIZATION OF 4-METHOXYSTYRENE INITIATED BY PYRYLIUM SALTS Andrew Perkowski A dissertation submitted to the faculty of the University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Chemistry. Chapel Hill 2014 Approved by: David A Nicewicz Erik Alexanian Marcey Waters Maurice Brookhart Joseph Templeton © 2014 Andrew Perkowski ALL RIGHTS RESERVED ii ABSTRACT Andrew Perkowski: Direct, Catalytic, Intermolecular Anti-Markovnikov Hydroacetoxylations of Alkenes Enabled via Photoredox Catalysis, and Investigations into the Mechanism of the Polymerization of 4-Methoxystyrene Initiated by Pyrylium Salts (Under the Direction of David A. Nicewicz) I. Intermolecular Anti-Markovnikov Addition of Oxygen Nucleophiles to Alkenes: Direct and Indirect Catalytic Methods to Address a Fundamental Challenge Markovnikov versus anti-Markovnikov functionalization of alkenes is discussed, as well as the inherent challenge in overcoming Markovnikov selectivity. Stoichiometric and catalytic methods for anti-Markovnikov functionalization are discussed and appraised. II. Anti-Markovnikov Addition of Oxygen Nucleophiles to Alkene Cation Radical Intermediates The formation of alkene cation radicals is disccused, as well as examples of anti- Markovnikov nucleophilic addition to alkene cation radicals. III. The Direct Anti-Markovnikov -

ABSTRACT Synthesis of New Chiral Pyrylium Salts, the Corresponding
ABSTRACT Synthesis of New Chiral Pyrylium Salts, the Corresponding Phosphinine and Pyridine Derivatives and the Kinetic Studies of the Epimerization of Pyrylium Salts Nelson A. van der Velde, Ph.D. Mentor: Charles M. Garner, Ph.D. Despite the versatility of pyrylium salts as precursors to many heteroaromatic systems, chiral pyrylium salts are almost unknown in the literature. One reason for this scarcity is that pyrylium salts are often involved as intermediates rather than as isolated and characterized materials. Another is that many pyrylium salts preparations tend to result in non-characterizable black solid due to polymerization reactions. We have developed the synthesis of several new chiral pyrylium salts and their conversion to the corresponding pyridines and phosphinines. This work almost triples the number of reported chiral pyrylium salts, and also represents the first racemizable/epimerizable pyrylium salts. The derived phosphinines and pyridines represent rare alpha-chiral ligands for transition metals. Interestingly, only a few examples of chiral phosphinines have been reported in the literature. Incorporation of chirality directly (i.e., alpha to aromatic ring) onto these planar ring systems has proven to be difficult. From our pyrylium salts we have synthesized new phosphinines with the chirality as close as possible to the phosphorus center. Two known pyridinium salts were also prepared with the thiosemicarbazone moiety. The cytotoxicity and inhibition of cruzain were evaluated and found to be non-actives. Our interest in chiral pyrylium salts led us to investigate the configurational stability of chiral centers alpha to the pyrylium ring. Although no epimerizable (or even racemizable) pyrylium salts have been reported, deuterium exchange at ortho and especially para benzylic positions is well-known, suggesting that epimerization is possible. -

Catalytic Synthesis of 1,3,5-Triphenylbenzenes, Β
J. Mex. Chem. Soc. 2006, 50(3), 114-118 Article © 2006, Sociedad Química de México ISSN 1870-249X Catalytic Synthesis of 1,3,5-Triphenylbenzenes, β-Methylchalcones and 2,4,6-Triphenyl Pyrylium Salts, Promoted by a Super Acid Triflouromethane Sulfonic Clay from Acetophenones Rosario Ruíz-Guerrero,1 Jorge Cárdenas,1 Lorena Bautista,1 Marina Vargas,1,3 Eloy Vázquez-Labastida2 and Manuel Salmón*1 1 Instituto de Química de la Universidad Nacional Autónoma de México, Circuito Exterior, Ciudad Universitaria, Coyoacán 04510, México D. F. [email protected] 2 Escuela Superior de Ingeniería Química e Industrias Extractivas del Instituto Politécnico Nacional, Edificio 7, Unidad Profesional, Zacatenco, Col. La Escalera, México 07038, D. F. 3 Departamento de Química, Facultad de Estudios Superiores Cuautitlán, Universidad Nacional Autónoma de México, Cuautitlán Izcalli, Estado de México, México 54740. Dedicated to Professor Pedro Joseph-Nathan on the occasion of his 65th birthday. Recibido el 9 de marzo del 2006; aceptado el 31 de mayo del 2006 Abstract. A montmorillonite clay from the State of Durango, Resumen. Una arcilla de montmorilonita del estado de Durango se Mexico, was used as catalyst. The clay was activated with trifluo- empleó como catalizador. La arcilla fue activada con ácido trifluoro- romethane sulfonic acid and used to study the condensation of differ- metansulfónico y usada para estudiar la condensación de diferentes ent acetophenones in refluxing benzene to produce triphenylben- acetofenonas en benceno a reflujo para producir trifenilbencenos, β- zenes, β-methylchalcones and pyrylium salts. This catalytic proce- metilchalconas y sales de pirilio. Este proceso catalítico produjo un dure afforded a new simple one-step method for the synthesis of com- nuevo método en una sola etapa para la síntesis de moléculas comple- plex organic molecules through the C-C bonds formation and the jas a través de la formación de enlaces C-C con el contraión del ácido counter ion for the pyrylium salt. -

On the Impact of Excited State Antiaromaticity Relief in a Fundamental
On the Impact of Excited State Antiaromaticity Relief in a Fundamental Benzene Photoreaction Leading to Substituted Bicyclo[3.1.0]hexenes Tomáš Slanina,1,2 Rabia Ayub,1,‡ Josene Toldo,1,‡ Johan Sundell,3 Wangchuk Rabten,1 Marco Nicaso,1 Igor Alabugin,4 Ignacio Fdez. Galván,5 Arvind K. Gupta,1 Roland Lindh,5,6 Andreas Orthaber,1 Richard J. Lewis,7 Gunnar Grönberg,7 Joakim Bergman3,* and Henrik Ottosson1,* 1 Department of Chemistry – Ångström Laboratory, Uppsala University, SE-751 20, Uppsala, Sweden. 2 Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences, Flemingovo námĕstí 2, 16610 Prague 6, Czech Republic 3 Medicinal Chemistry, Research and Early Development Cardiovascular, Renal and Metabolism, BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden. 4 Department of Chemistry and Biochemistry, Florida State University, Tallahassee, FL 32306-4390, USA, 5 Department of Chemistry – BMC, Uppsala University, 751 23, Uppsala, Sweden, 6 Uppsala Center for Computational 7 Chemistry – UC3, Uppsala University, 751 23, Uppsala Sweden, Medicinal Chemistry, Research and Early Development Respiratory, Inflammation and Autoimmune, BioPharmaceutical R&D, AstraZeneca Gothenburg, Sweden. ‡ These authors contributed equally. Table of contents 1. Materials and Methods ..................................................................................................................... 3 1.1 General information ..................................................................................................................... -
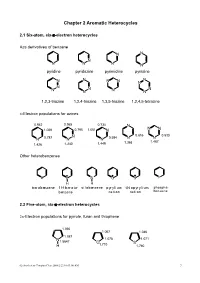
Chapter 2 Aromatic Heterocycles
Chapter 2 Aromatic Heterocycles 2.1 Six-atom, six-π-electron heterocycles Aza derivatives of benzene N N N N N N N pyridine pyridazine pyrimidine pyrazine N N N N N N N N N N N N N 1,2,3-triazine 1,2,4-triazine 1,3,5-triazine 1,2,4,5-tetrazine π-Electron populations for azines 0.942 0.965 0.734 N 1.029 0.795 1.051 N N N N 0.816 0.533 N 0.787 N N 0.584 N N 1.368 1.467 1.426 1.240 1.449 Other heterobenzenes B B Si O S P H H borabenzene 1H-borata- silabenzene pyrylium thiopyrylium phospha- benzene cation cation benzene 2.2 Five-atom, six-π-electron heterocycles 2π-Electron populations for pyrrole, furan and thiophene 1.090 1.067 1.046 1.087 N 1.078 1.071 1.5647 O 1.710 S H 1.760 02-Aro-hetero Chinpiao Chen 2008/2/22 10:03:00 AM 7 Aromatic five-membered heterocycles with two or more heteroatoms N N N N N N N N N N N O S S H H H isothiazole oxazole thiazole 1H-pyrazole 1H-imidazole 1H-tetrazole 2.3 Benzo-fused ring systems Benzo-fused six-membered nitrogen heteroaromatics N N N N N N quinoline isoquinoline cinnoline quinazoline N N N N N N N N N N quinoxaline phthalazine 1,2,3-benzotriazine 1,2,4-benzotriazine Five-membered benzo-fused heterocycles NH N O S H indole benzofuran benzo[b]thiophene isoindole N N O N N N O N H H isobenzofuran benzimidazole 1,2-benzisoxazole 1H-benzotriazole Other fused heterocycles N N N N N N N N N H N N phenanthridine pteridine purine quinolizinium cation indolizine 02-Aro-hetero Chinpiao Chen 2008/2/22 10:03:00 AM 8 2.4 Some criteria of aromaticity in heterocycles Bond lengths Typical lengths (Å) of single and -

Versatile Synthetic Methods for Photoluminescent Pyrylium Tosylates Jung Jae Koh University of Nevada, Las Vegas, [email protected]
UNLV Theses, Dissertations, Professional Papers, and Capstones 8-1-2015 Versatile Synthetic Methods for Photoluminescent Pyrylium Tosylates Jung Jae Koh University of Nevada, Las Vegas, [email protected] Follow this and additional works at: https://digitalscholarship.unlv.edu/thesesdissertations Part of the Chemistry Commons Repository Citation Koh, Jung Jae, "Versatile Synthetic Methods for Photoluminescent Pyrylium Tosylates" (2015). UNLV Theses, Dissertations, Professional Papers, and Capstones. 2487. https://digitalscholarship.unlv.edu/thesesdissertations/2487 This Thesis is brought to you for free and open access by Digital Scholarship@UNLV. It has been accepted for inclusion in UNLV Theses, Dissertations, Professional Papers, and Capstones by an authorized administrator of Digital Scholarship@UNLV. For more information, please contact [email protected]. VERSATILE SYNTHETIC METHODS FOR PHOTOLUMINESCENT PYRYLIUM TOSYLATES by Jung Jae Koh Bachelor of Biochemistry University of Nevada, Las Vegas 2011 A thesis submitted in partial fulfillment of the requirements for the Master of Science - Chemistry Department of Chemistry and Biochemistry College of Science The Graduate College University of Nevada, Las Vegas August 2015 Copyright by Jung Jae Koh, 2015 All Rights Reserved Thesis Approval The Graduate College The University of Nevada, Las Vegas July 21, 2015 This thesis prepared by Jung Jae Koh entitled Versatile Synthetic Methods for Photoluminescent Pyrylium Tosylates is approved in partial fulfillment of the requirements for the degree of Master of Science – Chemistry Department of Chemistry and Biochemistry Pradip K. Bhowmik, Ph.D. Kathryn Hausbeck Korgan, Ph.D. Examination Committee Co-Chair Graduate College Interim Dean Vernon F. Hodge, Ph.D. Examination Committee Co-Chair Dong-Chan Lee, Ph.D. -

Pyrylium Salts with Long Alkyl Substituents, II
Pyrylium Salts with Long Alkyl Substituents, II [1] 2,4-Dimethyl-6-undecylpyrylium Perchlorate and Derived Pyridinium Salts Mariana Bogatian, Calin Deleanu, Gheorghe Mihai, and Teodor Silviu Balaban* Center of Organic Chemistry of the Roumanian Academy Splaiul Independentei 202 B, 71141 Bucharest, PO 15/258, Roumania Z. Naturforsch. 47b, 1011-1015 (1992); received September 25, 1991/January 10, 1992 Pyrylium Salts, Pyridinium Salts, Synthetic Amphiphiles, NMR Spectra The crystalline title pyrylium salt was obtained by SnCl4 catalysed acylation of mesityl oxide with lauroyl chloride followed by treatment with perchloric acid and column chromatography. This new pyrylium salt was converted in high yields into the corresponding pyridine and N-substituted pyridinium salts (N-methyl, N-phenyl, N-(4-«-butylphenyl), and N-dodecyl) whose proton (300 MHz) and carbon-13 (75 MHz) NMR spectra are presented. Introduction eleven carbon a-alkyl side-chain and pyridinium One long aliphatic (lipophilic) chain attached to salts derived therefrom. strongly polar (hydrophilic) groups leads to com pounds with tensioactive properties. These are due either to micelar aggregates or to membranes. Two Results and Discussion long lipophilic chains attached to one polar group The acylation of mesityl oxide (1) was effected lead to amphiphiles, which can form bilayer mem with lauroyl chloride of comercial source, by branes [2]. Some such synthetic analogs of bio known methods [ 8], When A1C1 3 was used as acy- membranes have been prepared and their catalytic lating catalyst, the yields were low (<5% ) due to activity has been investigated and compared with the poor isolation of the desired title salt 2, while that of phospholipids and lecithins [3]. -

Machine Learning Accelerates the Discovery of Design Rules and Exceptions in Stable Metal–Oxo Intermediate Formation
Machine Learning Accelerates the Discovery of Design Rules and Exceptions in Stable Metal–Oxo Intermediate Formation The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Nandy, Aditya et al. "Machine Learning Accelerates the Discovery of Design Rules and Exceptions in Stable Metal–Oxo Intermediate Formation." ACS Catalysis 9, 9 (July 2019): 8243-8255 © 2019 American Chemical Society As Published http://dx.doi.org/10.1021/acscatal.9b02165 Publisher American Chemical Society (ACS) Version Final published version Citable link https://hdl.handle.net/1721.1/122278 Terms of Use Creative Commons Attribution-NonCommercial-NoDerivs License Detailed Terms http://creativecommons.org/licenses/by-nc-nd/4.0/ This is an open access article published under a Creative Commons Non-Commercial No Derivative Works (CC-BY-NC-ND) Attribution License, which permits copying and redistribution of the article, and creation of adaptations, all for non-commercial purposes. Research Article Cite This: ACS Catal. 2019, 9, 8243−8255 pubs.acs.org/acscatalysis Machine Learning Accelerates the Discovery of Design Rules and Exceptions in Stable Metal−Oxo Intermediate Formation Aditya Nandy,†,‡ Jiazhou Zhu,§ Jon Paul Janet,† Chenru Duan,†,‡ Rachel B. Getman,*,§ and Heather J. Kulik*,† † ‡ Department of Chemical Engineering and Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, United States § Department of Chemical & Biomolecular Engineering, Clemson University, Clemson, South Carolina 29634, United States *S Supporting Information ABSTRACT: Metal−oxo moieties are important catalytic intermediates in the selective partial oxidation of hydrocarbons and in water splitting. Stable metal−oxo species have reactive properties that vary depending on the spin state of the metal, complicating the development of structure−property relation- ships. -

Syntheses of Elusive Unsaturated Silacycles Gary Thomas Burns Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1981 Syntheses of elusive unsaturated silacycles Gary Thomas Burns Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Burns, Gary Thomas, "Syntheses of elusive unsaturated silacycles " (1981). Retrospective Theses and Dissertations. 7402. https://lib.dr.iastate.edu/rtd/7402 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This was produced from a copy of a document sent to us for microfilming. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help you understand markings or notations which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure you of complete continuity. 2. When an image on the film is obliterated with a round black mark it is an indication that the film inspector noticed either blurred copy because of movement during exposure, or duplicate copy. -

Zeitschrift Für Naturforschung / B / 52 (1997)
Band 52b Zeitschrift für Naturforschung 1997 Contents Contents of Number 1 Structural Investigation of Bis(isonitrile)gold(I) Complexes Original Communications W. Schneider, A. Sladek, A. Bauer, K. Anger- maier, H. Schmidbaur 53 Synthesis of New ll\4P-Diphosphapentalene- and lA5,5A5-Diphosphaazulene Systems Preparation and Spectroscopic Characterization of (In German) (^3-Hexahydro-c/c>50-hexaborato)phenyl- mercury(l-) [Hg(^3-B6H6)Ph]' and Crystal B. M erk, M. Fath, H. P r i t z k o w , H. P. L a t s c h a Structure of [PPh4][Hg(? 73-B6H 6)Ph] 1 (In German) Synthesis and Reactivity of y-Triphenylstannyl-a- T. Schaper, W. Preetz 57 aminobutyric Acid Derivatives (In German) Ligand Exchange Reactions of ReCl4(PPh3)2 with K. D ölling, A. Krug, H. Hartung, H. W eich- Salicylaldehyde-2-hydroxy(mercapto)anil. Mo M ANN 9 lecular Structure of Bis[salicylaldehyde-2-hy- droxyanilato(2-)]rhenium(IV) (In German) Coordination Chemistry of Lipoic Acid and Re S. Sawusch, U. Schilde, E. U h l e m a n n 61 lated Compounds, Part 1. Syntheses and Crystal Structures of the UV- and Light-Sensitive Li- Charge - Transfer Complexes of Metal Dithiolenes poato Complexes [M(lip)2(H20 )2] (M = Zn, Cd) XXIV: Ion Pairs of the Tetrakis(dimethylamino)- H. Strasdeit, A. von D öllen, A.-K. D u h m e 1 7 ethene Dication with Dithiolene Metalate Dia nions Photochemical Synthesis of New Tinorganic Com M . L e m k e , F. K n o c h , H. K is c h 65 pounds with Active Substituents on Tin (In German) Bis(trifluoromethyl)disulfane and Trisulfane: Mo lecular Geometry in the Solid State (In Ger T.