Niobium - Machining & Forming Operations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution and Understanding of the D-Block Elements in the Periodic Table Cite This: Dalton Trans., 2019, 48, 9408 Edwin C
Dalton Transactions View Article Online PERSPECTIVE View Journal | View Issue Evolution and understanding of the d-block elements in the periodic table Cite this: Dalton Trans., 2019, 48, 9408 Edwin C. Constable Received 20th February 2019, The d-block elements have played an essential role in the development of our present understanding of Accepted 6th March 2019 chemistry and in the evolution of the periodic table. On the occasion of the sesquicentenniel of the dis- DOI: 10.1039/c9dt00765b covery of the periodic table by Mendeleev, it is appropriate to look at how these metals have influenced rsc.li/dalton our understanding of periodicity and the relationships between elements. Introduction and periodic tables concerning objects as diverse as fruit, veg- etables, beer, cartoon characters, and superheroes abound in In the year 2019 we celebrate the sesquicentennial of the publi- our connected world.7 Creative Commons Attribution-NonCommercial 3.0 Unported Licence. cation of the first modern form of the periodic table by In the commonly encountered medium or long forms of Mendeleev (alternatively transliterated as Mendelejew, the periodic table, the central portion is occupied by the Mendelejeff, Mendeléeff, and Mendeléyev from the Cyrillic d-block elements, commonly known as the transition elements ).1 The periodic table lies at the core of our under- or transition metals. These elements have played a critical rôle standing of the properties of, and the relationships between, in our understanding of modern chemistry and have proved to the 118 elements currently known (Fig. 1).2 A chemist can look be the touchstones for many theories of valence and bonding. -
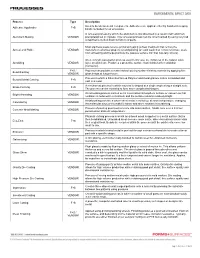
Process Type Description Adhesive Application F+S Used to Bond Non-Metal Components. Adhesives Are Applied Either by Hand Or In
Process Type Description Used to bond non-metal components. Adhesives are applied either by hand or in a spray Adhesive Application F+S booth controlled for air emissions. A two-step process by which the aluminum is first dissolved in a caustic bath and then Aluminum Making VENDOR precipitated out in crystals. This two-step process can be circumvented by using recycled scrap that is melted down to form new parts. Most stainless steels receive a final annealing (a heat treatment that refines the Anneal and Pickle VENDOR material's mechanical properties) and pickling (an acid wash that removes furnace scale from annealing and helps promote the passive surface film that naturally occurs). An electrolytic passivation process used to increase the thickness of the natural oxide Anodizing VENDOR layer on aluminum. Produces a protective surface that inhibits further oxidation (corrosion). F+S / Process that provides a matte/etched looking surface finish to material by applying fine Bead Blasting VENDOR glass beads at low pressure. Process in which a Fiber-Reinforced Polymer and metal granule matrix is blended and Bonded Metal Casting F+S cast in a mold. A mechanical process in which material is shaped at a single angle along a straight axis. Brake Forming F+S The process can be repeated to form more complicated shapes. An annealing process carried out in a controlled atmosphere furnace or vacuum so that Bright Annealing VENDOR oxidation is reduced to a minimum and the surface remains relatively bright. Metalworking process in which sheet metal is rolled out at room temperature, changing Calendaring VENDOR the molecular structure to make it harder and more resistant to scratching. -

Development of Niobium-Boron Grain
DEVELOPMENT OF NIOBIUM-BORON GRAIN REFINER FOR ALUMINIUM-SILICON ALLOYS A thesis submitted for the degree of Doctor of Philosophy (PhD) by Magdalena Nowak Brunel Centre for Advanced Solidification Technology (BCAST) Brunel University September 2011 To my mother and my late father... II Abstract Aluminium castings with a large grain structure have poor mechanical properties which are primarily due to casting defects as opposed to fine grain structure. The grain refinement practice using chemical addition is well established for wrought alloys, however in the case of casting alloys, the practice of adding grain refiners and the impact on castability is not well established. The addition of well known Al-5Ti-B grain refiner to casting alloys with silicon (Si) content above 3 wt.% is not effective. This is believed to be due to the chemical reaction between Ti and Si. The current research aim is to find an alternative, but effective, chemical phase which can refine Al-Si alloy grains. Based on a crystallographic database search and intermetallic phases found in Aluminium–Niobium-Boron, there exists several iso-structural phases similar to those of Al3Ti and TiB2. We have selected a phase which exhibits chemical phase stability with Si (below 900 oC) and developed a potential novel grain refiner Nb-B for Al-Si cast alloys. Various Al-Si binary alloys and a commercial sourced LM6 (Al-10Si-Mg) cast alloys were cast after novel grain refiner addition to the melt. It is the first time that such fine grain structures were achieved for Al-Si alloys when Si >4wt.%. -

Stable Isotopes of Niobium Properties of Niobium
Stable Isotopes of Niobium Isotope Z(p) N(n) Atomic Mass Natural Abundance Nuclear Spin Nb-93 41 52 92.906376 100% 9/2+ Niobium was discovered in 1801 by Charles Hatchett. Its name comes from the Greek name Niobe, meaning “daughter of Tantalus” (tantalum is closely related to niobium in the periodic table of elements). Because the niobium was discovered in an ore called columbite, it was known temporarily as columbium. Niobium, a gray or silvery soft metal, is ductile and very malleable at room temperature and does not tarnish or oxidize at room temperature. It only reacts with oxygen and halogens when heated. It is less corrosion- resistant than tantalum is at high temperatures. Niobium is not attacked by nitric acid up to 100 °C but is vigorously attacked by the mixture of nitric and hydrofluoric acids. It is unaffected at room temperature by most acids and by aqua regia. It is attacked by alkaline solutions, to some extent, at all temperatures. Niobium becomes a superconductor at 9.15 °K. It is insoluble in water, hydrochloric acid, nitric acid and aqua regia; soluble in hydrofluoric acid; and soluble in fused alkali hydroxide. At ordinary temperatures niobium does not react with most chemicals; however, the metal is slowly attacked by hydrofluoric acid and dissolves and is attacked by hydrogen fluoride and fluorine gases, forming niobium pentafluoride. Niobium is oxidized by air at 350 ºC, first forming a pale yellow oxide film of increasing thickness, which changes its color to blue. On further heating to 400 ºC, it converts to a black film of niobium dioxide. -

Separation of Niobium and Tantalum - a Literature Survey Ernest L
Ames Laboratory ISC Technical Reports Ames Laboratory 8-15-1956 Separation of niobium and tantalum - a literature survey Ernest L. Koerner Iowa State College Morton Smutz Iowa State College Follow this and additional works at: http://lib.dr.iastate.edu/ameslab_iscreports Part of the Chemistry Commons Recommended Citation Koerner, Ernest L. and Smutz, Morton, "Separation of niobium and tantalum - a literature survey" (1956). Ames Laboratory ISC Technical Reports. 145. http://lib.dr.iastate.edu/ameslab_iscreports/145 This Report is brought to you for free and open access by the Ames Laboratory at Iowa State University Digital Repository. It has been accepted for inclusion in Ames Laboratory ISC Technical Reports by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Separation of niobium and tantalum - a literature survey Abstract During recent years increased interest has been shown in the metal niobium. Because niobium has a high melting point (4376° F) and a low neutron cross-section, the Atomic Energy Commission has encouraged research on new methods of winning the metal from its ores with the ultimate hope of finding a process which would yield niobium metal at a reasonable price. The two areas of endeavor in which·the principal difficulties in finding such an economic process occur are the separation of tantalum from the niobium and the conversion of purified niobium salts to the metal. This report, then, was written to consolidate the more important information available in the literature on one of these troublesome areas, that of the separation of niobium and tantalum. -

Effect of Oxygen on the Corrosion of Niobium and Tantalum by Liquid Lithium
ORNL-TM-4069 DECEIVED BY TIC MAR 2 01973 THE EFFECT OF OXYGEN ON THE CORROSION OF NIOBIUM AND TANTALUM BY LIQUID LITHIUM R. L. Klueh MASTER SiSTiHBUTWfl c-f THIS &3CmMT 13 U&UMITED r. I . • -I ' / •''Hi'; 1 . V/', •• I • • H I •-' , C •!", >i ; ' o ': 'J -1 ; .•..'.' •-•'I' < . • . .J ,• ! OAK RIDGE NATIONAL LABORATORY '-I. ' ' t- /operated'by "unjon 'carbide, coloration • forjhe'u.s. atowic1 ENERGY'CQMMISSION 1 • - " <•:-.*- '' .1 iS> '•'• . ' , 1 <• \ .1 I . 'v: • = 1 r i. H. ORNL-TM-4069 Contract No. W-7405-eng-26 METALS AND CERAMICS DIVISION THE EFFECT OF OXYGEN ON THE CORROSION OF NIOBIUM AND TANTALUM BY LIQUID LITHIUM R. L. Klueh NOTICE This report was prepared as an account of work sponsored by the United States Government. Neither the United States noi' the United States Atomic Energy Commission, nor any of their employees, nor any of their contractors, subcontractors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility fir the accuracy, com- pleteness or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. MARCH 1973 OAK RIDGE NATIONAL LABORATORY Oak Ridge, Tennessee 37830 operated by • i UNION CARBIDE CORPORATION for the U.S. ATOMIC ENERGY COMMISSION MASTER • • • 11X CONTENTS Page Abstract 1 Introduction 1 Experimental 5 Results ...... 7 The Effect of Oxygen in Lithium 7 The Effect of Oxygen in the Refractory Metal 9 Discussion ........ 16 Acknowledgments 21 THE EFFECT OF OXYGEN ON THE CORROSION OF NIOBIUM AND TANTALUM BY LIQUID LITHIUM R. L. -

Niobium Pentoxide Based Materials for High Rate Rechargeable Electrochemical Energy Storage Cite This: Mater
Materials Horizons View Article Online REVIEW View Journal | View Issue Niobium pentoxide based materials for high rate rechargeable electrochemical energy storage Cite this: Mater. Horiz., 2021, 8, 1130 Fei Shen,a Zhongti Sun,ab Qinggang He, *c Jingyu Sun, abd Richard B. Kaner *e and Yuanlong Shao*abd The demand for high rate energy storage systems is continuously increasing driven by portable electronics, hybrid/electric vehicles and the need for balancing the smart grid. Accordingly, Nb2O5 based materials have gained great attention because of their fast cation intercalation faradaic charge storage that endows them with high rate energy storage performance. In this review, we describe the crystalline features of the five main phases of Nb2O5 and analyze their specific electrochemical characteristics with an emphasis on the intrinsic ion intercalation pseudocapacitive behavior of T-Nb2O5. The charge Received 14th September 2020, storage mechanisms, electrochemical performance and state-of-the-art characterization techniques for Accepted 9th December 2020 Nb2O5 anodes are summarized. Next, we review recent progress in developing various types of Nb2O5 DOI: 10.1039/d0mh01481h based fast charging electrode materials, including Nb2O5 based mixed metal oxides and composites. Finally, we highlight the major challenges for Nb2O5 based materials in the realm of high rate rechargeable rsc.li/materials-horizons energy storage and provide perspectives for future research. 1. Introduction Due to the increasing demand for rapid charging and high power a College of Energy, Soochow Institute for Energy and Materials InnovationS delivery for portable electronics and electricvehicles,highrate (SIEMIS), Jiangsu Provincial Key Laboratory for Advanced Carbon Materials and energy storage systems (ESSs) that can store/release charge in Wearable Energy Technologies, Soochow University, 215006 Suzhou, P. -

Hand Spinning and CNC Spinning
Hand Spinning and CNC Spinning 931 N. Ridge Avenue, Lombard, IL 60148 Phone: 630.268.9292 • Fax: 630.268.9393 [email protected] • www. helandermetal.com Metal Spinning, a Bridge Between Craft and Automation Metal Spinning is a metalworking process that stands firmly between the artisans and craftsmen of the past and the machine tool automation of the present and future. It is a process certain to benefit from the high volume manufacturing techniques being developed, while still demanding high levels of individual craftsmanship. Manufacturers choosing to work in metal spinning will tap into the high production capabilities of an automated shop floor, but also require manual spinning to create more intricate architectural and decorative products. Combining both of these techniques allows for the mass production of the bulk of a product line through CNC automation, while finishing it up with hand spinning creating a product that is hand-worked. Metal Spinning Technology: A Closer Look On the surface, the spinning process is a simple one. A formed block, or mandrel, is mounted in the center drive section of a lathe. This formed block is the part that defines the shape of the final product. The block is machined from a variety of materials, including wood, polyurethane, aluminum and steel, each having its own strengths and weaknesses. A pre-sized metal disk, called the work piece, is then clamped against the ‘front’ of the block by a pressure pad. This pressure pad is also attached to the tailstock of the lathe. The block and the attached work piece are then rotated at high speeds. -

PDH Course M381
PDHonline Course M 497 (6 PDH) _______________________________________________________________________________________ Conventional Machining Technology Fundamentals Instructor: Jurandir Primo, PE 2013 PDH Online | PDH Center 5272 Meadow Estates Drive Fairfax, VA 22030-6658 Phone & Fax: 703-988-0088 www.PDHonline.org www.PDHcenter.com An Approved Continuing Education Provider www.PDHcenter.com PDH Course M 497 www.PDHonline.org CONVENTIONAL MACHINING TECHNOLOGY – FUNDAMENTALS Introduction Shaping Machines Lathes Slotting Machines - Metalworking lathes - Planing, shaping and slotting calculations - Classification of lathes - Turning operations Boring Machines - Semiautomatic and automatic lathes - Types of boring machines - Accessories - Boring types - Live centers and dead centers - Boring calculations - Rests and micrometer supports - Lathe cutting tools Hobbing & Gear Shaping Machines - Lathe calculations - Common gear generation types - Graduate micrometer and measurements - Details of involute gearing - Tools and inserts - Proper meshing and contact ratio - Common holders with inserts - Gear Shaping Machines - Goose-neck holders with inserts Broaching Machines Drilling Machines - Horizontal broaching machines - Classification of drilling machines - Vertical broaching machines - Application of drilling machines - Broaching principles - Types of drills - Broaching configuration - Drill sizes and geometry - Materials of broaches - Drill point angles - Geometry of broaching teeth - Drill holding & clamping of workpieces - Broaching operations -

Manufacturing Technology I Unit I Metal Casting
MANUFACTURING TECHNOLOGY I UNIT I METAL CASTING PROCESSES Sand casting – Sand moulds - Type of patterns – Pattern materials – Pattern allowances – Types of Moulding sand – Properties – Core making – Methods of Sand testing – Moulding machines – Types of moulding machines - Melting furnaces – Working principle of Special casting processes – Shell – investment casting – Ceramic mould – Lost Wax process – Pressure die casting – Centrifugal casting – CO2 process – Sand Casting defects. UNIT II JOINING PROCESSES Fusion welding processes – Types of Gas welding – Equipments used – Flame characteristics – Filler and Flux materials - Arc welding equipments - Electrodes – Coating and specifications – Principles of Resistance welding – Spot/butt – Seam – Projection welding – Percusion welding – GS metal arc welding – Flux cored – Submerged arc welding – Electro slag welding – TIG welding – Principle and application of special welding processes – Plasma arc welding – Thermit welding – Electron beam welding – Friction welding – Diffusion welding – Weld defects – Brazing – Soldering process – Methods and process capabilities – Filler materials and fluxes – Types of Adhesive bonding. UNIT III BULK DEFORMATION PROCESSES Hot working and cold working of metals – Forging processes – Open impression and closed die forging – Characteristics of the process – Types of Forging Machines – Typical forging operations – Rolling of metals – Types of Rolling mills – Flat strip rolling – Shape rolling operations – Defects in rolled parts – Principle of rod and wire drawing – Tube drawing – Principles of Extrusion – Types of Extrusion – Hot and Cold extrusion – Equipments used. UNIT IV SHEET METAL PROCESSES Sheet metal characteristics – Typical shearing operations – Bending – Drawing operations – Stretch forming operations –– Formability of sheet metal – Test methods – Working principle and application of special forming processes – Hydro forming – Rubber pad forming – Metal spinning – Introduction to Explosive forming – Magnetic pulse forming – Peen forming – Super plastic forming. -

(Nb-Ti) Superconducting Joints for Persistent-Mode Operation
www.nature.com/scientificreports OPEN Niobium-titanium (Nb-Ti) superconducting joints for persistent-mode operation Received: 21 June 2019 Dipak Patel1,2, Su-Hun Kim1,3, Wenbin Qiu1, Minoru Maeda4, Akiyoshi Matsumoto2, Accepted: 26 August 2019 Gen Nishijima2, Hiroaki Kumakura2, Seyong Choi4 & Jung Ho Kim1 Published: xx xx xxxx Superconducting joints are essential for persistent-mode operation in a superconducting magnet system to produce an ultra-stable magnetic feld. Herein, we report rationally designed niobium- titanium (Nb-Ti) superconducting joints and their evaluation results in detail. For practical applications, superconducting joints were fabricated by using a solder matrix replacement method with two types of lead-bismuth (Pb-Bi) solder, including Pb42Bi58 as a new composition. All the joints attained a critical current of >200 A below 1.43 T at 4.2 K. Our optimized superconducting joining method was tested in a closed-loop coil, obtaining a total circuit resistance of 3.25 × 10−14 Ω at 4.2 K in self-feld. Finally, persistent-mode operation was demonstrated in an Nb-Ti solenoid coil with a persistent-current switch. This work will pave the way to developing high-performance Nb-Ti superconducting joints for practical applications. Superconducting magnets are used to produce a strong magnetic feld in a compact system, which otherwise cannot be attained using conventional copper (Cu) based magnets. Niobium-titanium (Nb-Ti) with a critical temperature (Tc) of 9.2 K is usually used to fabricate superconducting magnets for various practical applications. One of the distinct features of Nb-Ti that makes it ideal for most commercial applications is the possibility to produce reliable “superconducting joints”. -

Future Possibilities – Technology and the Market Place
NIOBIUM: FUTURE POSSIBILITIES – TECHNOLOGY AND THE MARKET PLACE Friedrich Heisterkamp(1) and Tadeu Carneiro(2) (1) Niobium Products Company GmbH, Steinstrasse 28, D-40210 Düsseldorf 1, Germany. (2) Reference Metals Company, Inc.,1000 Old Pond Road, Bridgeville, PA 15017-0217, U.S.A. Abstract Although niobium is now two hundred years old, it has been only during the past four decades that it has been consumed as an industrial material. During this time, niobium has been established as part of the engineering solution in numerous cases for its own technical merits. The most important of these cases are in the field of oil and gas transportation, automotive industry, high-rise buildings, aircraft engines and medical diagnosis devices. The future will see niobium as an increasingly important technological solution in a broad spectrum of end- uses as specific niobium properties continue to be investigated. The bulk of niobium will continue to go into steel since its present position is unchallenged in the three main microalloyed steel domains (pipe, automotive and structurals). In pipe steels niobium will remain a chief microalloying element in all steel grades. In automotive steels it will remain the most important microalloying element in all hot and cold rolled HSLA steels with a good chance to penetrate the new and important group of multi-phase steels. In addition, it is expected that all thermomechanically processed high strength steels for structural components will contain niobium as the first microalloy choice in the future. With regard to the non-ferrous applications niobium will be rather used for high value-added products.