Degenerative Lumbar Spondylosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Audit of Bone Mineral Density and Associated Factors in Patients With
Review Article Clinician’s corner Images in Medicine Experimental Research Case Report Miscellaneous Letter to Editor DOI: 10.7860/JCDR/2019/39690.12544 Original Article Postgraduate Education An Audit of Bone Mineral Density and Case Series Associated Factors in Patients with Orthopaedics Section Lumbar Spinal Stenosis Short Communication ARASH RAHBAR1, RAHMATOLLAH JOKAR2, SEYED MOKHTAR ESMAEILNEJAD-GANJI3 ABSTRACT Results: Overall, 146 patients with lumbar stenosis were Introduction: Osteoporosis is a major global health problem enrolled. Based on bone densitometry of spine and femur, and is commonly observed with lumbar stenosis in older 35 (24%) and 36 (24.7%) of the patients had osteoporosis. people. It is stated that osteoporosis may cause progressive According to femoral densitometry, age (OR=1.311, 95% CI: spinal deformities and stenosis in elderly patients. 1.167-1.473), being a female (OR=3.391, 95% CI: 1.391-8.420) and being a homemaker (OR=3.675, 95% CI: 1.476-9.146) Aim: To audit prevalence of low bone mineral density and were found as risk factors for osteoporosis. Based on spinal associated factors in patients with lumbar spinal stenosis. densitometry, age (OR=1.283, 95% CI: 1.154-1.427) and being Materials and Methods: Patients with symptomatic lumbar a female (OR=2.786, 95% CI: 1.106-7.019) were associated with spinal stenosis were recruited in this cross-sectional study, osteoporosis. Significant correlations were observed between who had been referred to Shahid Beheshti hospital in Babol, bone mineral density and red blood cell counts (r=+0.168, Northern Iran, between 2016 and 2017. -

Facet Joint Pathology
CLINICAL Facet Joint Pathology REVIEW Indexing Metadata/Description › Title/condition: Facet Joint Pathology › Synonyms: Facet joint syndrome; zygapophyseal joint pathology; facet joint arthropathy › Anatomical location/body part affected: Spine, specifically facet joint/s › Area(s) of specialty: Orthopedic rehabilitation › Description • Facet joints(1) –Fall under the category of synovial joints –Also called zygapophyseal joints • Types of facet joint pathology(1) –Sprain –Trauma to the capsule –Degenerative joint disease/osteoarthritis –Rheumatoid arthritis –Impingement - Pain and spasm result upon injury to the meniscoid - Generally occurs when the individual completes a quick or atypical movement - The movement typically entails spinal flexion and rotation –Prevalence of facet joint pathology has been estimated to be between 15% and 45% in patients with chronic low back pain(29) › ICD-9 codes • 724.8 other symptoms referable to back [facet syndrome] › ICD-10 codes • M24.8 other specific joint derangements, not elsewhere classified • M53.8 other specified dorsopathies • M54.5 low back pain Authors • M54.8 other dorsalgia Amy Lombara, PT, DPT • optional subclassification to indicate site of involvement for M53 and M54 Ellenore Palmer, BScPT, MSc –0 multiple sites in spine Cinahl Information Systems, Glendale, CA –5 thoracolumbar region Reviewers –6 lumbar region Rudy Dressendorfer, BScPT, PhD –7 lumbosacral region Cinahl Information Systems, Glendale, CA –8 sacral and sacrococcygeal region Lynn Watkins, BS, PT, OCS –9 site unspecified -

Juvenile Spondyloarthropathies: Inflammation in Disguise
PP.qxd:06/15-2 Ped Perspectives 7/25/08 10:49 AM Page 2 APEDIATRIC Volume 17, Number 2 2008 Juvenile Spondyloarthropathieserspective Inflammation in DisguiseP by Evren Akin, M.D. The spondyloarthropathies are a group of inflammatory conditions that involve the spine (sacroiliitis and spondylitis), joints (asymmetric peripheral Case Study arthropathy) and tendons (enthesopathy). The clinical subsets of spondyloarthropathies constitute a wide spectrum, including: • Ankylosing spondylitis What does spondyloarthropathy • Psoriatic arthritis look like in a child? • Reactive arthritis • Inflammatory bowel disease associated with arthritis A 12-year-old boy is actively involved in sports. • Undifferentiated sacroiliitis When his right toe starts to hurt, overuse injury is Depending on the subtype, extra-articular manifestations might involve the eyes, thought to be the cause. The right toe eventually skin, lungs, gastrointestinal tract and heart. The most commonly accepted swells up, and he is referred to a rheumatologist to classification criteria for spondyloarthropathies are from the European evaluate for possible gout. Over the next few Spondyloarthropathy Study Group (ESSG). See Table 1. weeks, his right knee begins hurting as well. At the rheumatologist’s office, arthritis of the right second The juvenile spondyloarthropathies — which are the focus of this article — toe and the right knee is noted. Family history is might be defined as any spondyloarthropathy subtype that is diagnosed before remarkable for back stiffness in the father, which is age 17. It should be noted, however, that adult and juvenile spondyloar- reported as “due to sports participation.” thropathies exist on a continuum. In other words, many children diagnosed with a type of juvenile spondyloarthropathy will eventually fulfill criteria for Antinuclear antibody (ANA) and rheumatoid factor adult spondyloarthropathy. -

Nonoperative Treatment of Lumbar Spinal Stenosis with Neurogenic Claudication a Systematic Review
SPINE Volume 37, Number 10, pp E609–E616 ©2012, Lippincott Williams & Wilkins LITERATURE REVIEW Nonoperative Treatment of Lumbar Spinal Stenosis With Neurogenic Claudication A Systematic Review Carlo Ammendolia , DC, PhD, *†‡ Kent Stuber, DC, MSc , § Linda K. de Bruin , MSc , ‡ Andrea D. Furlan, MD, PhD , ||‡¶ Carol A. Kennedy, BScPT, MSc , ‡#** Yoga Raja Rampersaud, MD , †† Ivan A. Steenstra , PhD , ‡ and Victoria Pennick, RN, BScN, MHSc ‡‡ or methylcobalamin, improve walking distance. There is very low- Study Design. Systematic review. quality evidence from a single trial that epidural steroid injections Objective. To systematically review the evidence for the improve pain, function, and quality of life up to 2 weeks compared effectiveness of nonoperative treatment of lumbar spinal stenosis with home exercise or inpatient physical therapy. There is low- with neurogenic claudication. quality evidence from a single trial that exercise is of short-term Summary of Background Data. Neurogenic claudication benefi t for leg pain and function compared with no treatment. There can signifi cantly impact functional ability, quality of life, and is low- and very low-quality evidence from 6 trials that multimodal independence in the elderly. nonoperative treatment is less effective than indirect or direct Methods. We searched CENTRAL, MEDLINE, EMBASE, CINAHL, surgical decompression with or without fusion. and ICL databases up to January 2011 for randomized controlled Conclusion. Moderate- and high-GRADE evidence for nonopera- trials published in English, in which at least 1 arm provided tive treatment is lacking and thus prohibiting recommendations to data on nonoperative treatments. Risk of bias in each study was guide clinical practice. Given the expected exponential rise in the independently assessed by 2 reviewers using 12 criteria. -
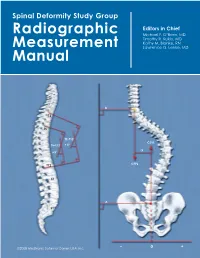
Spinal Deformity Study Group
Spinal Deformity Study Group Editors in Chief Radiographic Michael F. O’Brien, MD Timothy R. Kuklo, MD Kathy M. Blanke, RN Measurement Lawrence G. Lenke, MD Manual B T2 T5 T2–T12 CSVL T5–T12 +X° -X +X° C7PL T12 L2 A S1 ©2008 Medtronic Sofamor Danek USA, Inc. – 0 + Radiographic Measurement Manual Editors in Chief Michael F. O’Brien, MD Timothy R. Kuklo, MD Kathy M. Blanke, RN Lawrence G. Lenke, MD Section Editors Keith H. Bridwell, MD Kathy M. Blanke, RN Christopher L. Hamill, MD William C. Horton, MD Timothy R. Kuklo, MD Hubert B. Labelle, MD Lawrence G. Lenke, MD Michael F. O’Brien, MD David W. Polly Jr, MD B. Stephens Richards III, MD Pierre Roussouly, MD James O. Sanders, MD ©2008 Medtronic Sofamor Danek USA, Inc. Acknowledgements Radiographic Measurement Manual The radiographic measurement manual has been developed to present standardized techniques for radiographic measurement. In addition, this manual will serve as a complimentary guide for the Spinal Deformity Study Group’s radiographic measurement software. Special thanks to the following members of the Spinal Deformity Study Group in the development of this manual. Sigurd Berven, MD Hubert B. Labelle, MD Randal Betz, MD Lawrence G. Lenke, MD Fabien D. Bitan, MD Thomas G. Lowe, MD John T. Braun, MD John P. Lubicky, MD Keith H. Bridwell, MD Steven M. Mardjetko, MD Courtney W. Brown, MD Richard E. McCarthy, MD Daniel H. Chopin, MD Andrew A. Merola, MD Edgar G. Dawson, MD Michael Neuwirth, MD Christopher DeWald, MD Peter O. Newton, MD Mohammad Diab, MD Michael F. -

Double Spinal Cord Injury in a Patient with Ankylosing Spondylitis
Spinal Cord (1999) 37, 305 ± 307 ã 1999 International Medical Society of Paraplegia All rights reserved 1362 ± 4393/99 $12.00 http://www.stockton-press.co.uk/sc Case Report Double spinal cord injury in a patient with ankylosing spondylitis MN Akman*,1 M KaratasÎ1, SÎ KilincË 1 and M AgÏ ildere1 1Department of Physical Medicine and Rehabilitation and Radiology, BahcË elievler, Ankara, Turkey Ankylosing spondylitis patients are more prone to spinal fractures and these fractures commonly result in mobile nonunion. We report a patient with a 30-year history of ankylosing spondylitis who sustained double spinal cord injuries following minor trauma. The ®rst injury occurred at the lumbar level due to pseudoarthrosis of an old fracture, and the second at the thoracic level following cardiopulmonary arrest and an episode of hypotension. The possible mechanisms of the injuries are discussed and maintaining normal blood pressure in these patients is emphasized. Keywords: spinal cord injury; ankylosing spondylitis; spinal cord infarction; spinal fractures Introduction Ankylosing spondylitis (AS) has a prevalance of 1 per diagnostic workup. His arterial blood pressure stayed 1000 in the general population and primarily involves below normal and his central venous pressure remained 1 the vertebral column. Spinal rigidity due to long- below 5 cmH2O for about 12 h. ECG, chest X-Ray standing AS renders the patient susceptible to vertebral and cranial computed tomography (CT) were normal. trauma, so that even minor trauma may cause When the patient awoke and was in a stable condition, fractures.2±9 There are only a few reports in the he could not feel or move his legs. -

New ASAS Criteria for the Diagnosis of Spondyloarthritis: Diagnosing Sacroiliitis by Magnetic Resonance Imaging 9
Document downloaded from http://www.elsevier.es, day 10/02/2016. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. Radiología. 2014;56(1):7---15 www.elsevier.es/rx UPDATE IN RADIOLOGY New ASAS criteria for the diagnosis of spondyloarthritis: ଝ Diagnosing sacroiliitis by magnetic resonance imaging ∗ M.E. Banegas Illescas , C. López Menéndez, M.L. Rozas Rodríguez, R.M. Fernández Quintero Servicio de Radiodiagnóstico, Hospital General Universitario de Ciudad Real, Ciudad Real, Spain Received 17 January 2013; accepted 10 May 2013 Available online 11 March 2014 KEYWORDS Abstract Radiographic sacroiliitis has been included in the diagnostic criteria for spondy- Sacroiliitis; loarthropathies since the Rome criteria were defined in 1961. However, in the last ten years, Diagnosis; magnetic resonance imaging (MRI) has proven more sensitive in the evaluation of the sacroiliac Magnetic resonance joints in patients with suspected spondyloarthritis and symptoms of sacroiliitis; MRI has proven imaging; its usefulness not only for diagnosis of this disease, but also for the follow-up of the disease and Axial spondy- response to treatment in these patients. In 2009, The Assessment of SpondyloArthritis inter- loarthropathies national Society (ASAS) developed a new set of criteria for classifying and diagnosing patients with spondyloarthritis; one important development with respect to previous classifications is the inclusion of MRI positive for sacroiliitis as a major diagnostic criterion. This article focuses on the radiologic part of the new classification. We describe and illustrate the different alterations that can be seen on MRI in patients with sacroiliitis, pointing out the limitations of the technique and diagnostic pitfalls. -

Lumbar Spinal Stenosis a Patient's Guide to Lumbar Spinal Stenosis
Lumbar Spinal Stenosis A Patient's Guide to Lumbar Spinal Stenosis See a Washington Post story about a woman whose worsening pain stumped specialist after specialist for five years until she saw UM spine surgeon Steven Ludwig, who diagnosed the cause as spinal stenosis and performed successful surgery. Introduction Spinal stenosis is term commonly used to describe a narrowing of the spinal canal. This problem is much more common in people over the age of 60. However, it can occur in younger people who have abnormally small spinal canals as a type of birth defect. The problem usually causes back pain and leg pain that comes and goes with activities such as walking. The purpose of this information is to help you understand: • The anatomy of the spine relating to spinal stenosis • The signs and symptoms of lumbar spinal stenosis • How the condition is diagnosed • The treatments available for the condition Anatomy In order to understand your symptoms and treatment choices, you should start with some understanding of the general anatomy of your lumbar spine (lower back). This includes becoming familiar with the various parts that make up the spine and how these parts work together. Please review the document entitled: • Anatomy and Function of the Spine Causes Although there is some space between the spinal cord and the edges of the spinal canal, this space can be reduced by many conditions. Bone and tough ligaments surround the spinal canal. This tube cannot expand if the spinal cord or nerves require more space. If anything begins to narrow the spinal canal, the risk of irritation and injury of the spinal cord or nerves increases. -

Cervical Spondylosis
Page 1 of 6 Cervical Spondylosis This leaflet is aimed at people who have been told they have cervical spondylosis as a cause of their neck symptoms. Cervical spondylosis is a 'wear and tear' of the vertebrae and discs in the neck. It is a normal part of ageing and does not cause symptoms in many people. However, it is sometimes a cause of neck pain. Symptoms tend to come and go. Treatments include keeping the neck moving, neck exercises and painkillers. In severe cases, the degeneration may cause irritation or pressure on the spinal nerve roots or spinal cord. This can cause arm or leg symptoms (detailed below). In these severe cases, surgery may be an option. Understanding the neck The back of the neck includes the cervical spine and the muscles and ligaments that surround and support it. The cervical spine is made up of seven bones called vertebrae. The first two are slightly different to the rest, as they attach the spine to the skull and allow the head to turn from side to side. The lower five cervical vertebrae are roughly cylindrical in shape - a bit like small tin cans - with bony projections. The sides of the vertebrae are linked by small facet joints. Between each of the vertebrae is a 'disc'. The discs are made of a tough fibrous outer layer and a softer gel-like inner part. The discs act like 'shock absorbers' and allow the spine to be flexible. Strong ligaments attach to adjacent vertebrae to give extra support and strength. Various muscles attached to the spine enable the spine to bend and move in various ways. -

Chapter 4: Massage and Sciatica: an In-Depth Study 2 CE Hours
Chapter 4: Massage and Sciatica: An In-Depth Study 2 CE Hours By: Kerry Davis, LMT, CIMT, CPT Learning objectives Define the characteristics of sciatica. Discuss how to construct a treatment plan. Recognize the causes of sciatica. Discuss how to assess the client’s posture and gait. Compare sciatica with other conditions of the low back. Describe the evaluation of the client’s pain patterns and symptoms. Distinguish the muscle imbalance patterns attributing to sciatica. Demonstrate practice of test assessments to rule out other Understand the pattern of referred pain resulting from sciatica. conditions of the low back. Illustrate application of massage techniques to treat the client. Overview Low back pain affects more than three million people in the United encounter multiple cases during the course of their practice due to the States each year (Werner, 2002). According to a 2010 survey, low back impact that low back pain has on society. This course will educate the pain was listed as the third most oppressive condition afflicting people. massage therapist about how to identify sciatica. It will also familiarize Low back pain does not discriminate between men and women and the therapist with the most common causes of sciatica, discuss usually presents as early as the age of thirty; in fact, the prevalence differences between sciatica from piriformis syndrome and sacroiliac increases in correlation with age (National Institute of Neurological joint dysfunctions, examine the proper evaluation of the condition, as Disorders and Stroke, 2015). It is likely that massage therapists will well as develop the treatment protocols for sciatica. UNDERSTANDING SCIATICA Sciatica, or lumbar radiculopathy, is characterized as an inflammation in the feet and toes. -

Long-Term Follow-Up Review of Patients Who Underwent Laminectomy for Lumbar Stenosis: a Prospective Study
Long-term follow-up review of patients who underwent laminectomy for lumbar stenosis: a prospective study Manucher J. Javid, M.D., and Eldad J. Hadar, M.D. Department of Neurological Surgery, University of Wisconsin Hospital and Clinics, Madison, Wisconsin Object. Decompressive laminectomy for stenosis is the most common operation performed on the lumbar spine in older patients. This prospective study was designed to evaluate long-term results in patients with symptomatic lumbar stenosis. Methods. Between January 1984 and January 1995, 170 patients underwent surgery for lumbar stenosis (86 patients), lumbar stenosis and herniated disc (61 patients), or lateral recess stenosis (23 patients). The male/female ratio for each group was 43:43, 39:22, and 14:9, respectively. The average age for all groups was 61.4 years. For patients with lumbar stenosis, the success rate was 88.1% at 6 weeks and 86.7% at 6 months. For patients with lumbar stenosis and herniated disc, the success rate was 80% at 6 weeks and 77.6% at 6 months, with no statistically significant difference between the two groups. For patients with lateral recess stenosis, the success rate was 58.7% at 6 weeks and 63.6% at 6 months; however, the sample was not large enough to be statistically significant. One year after surgery a questionnaire was sent to all patients; 163 (95.9%) responded. The success rate in patients with stenosis had declined to 69.6%, which was significant (p = 0.012); the rate for patients with stenosis and herniated disc was 77.2%; and that for lateral recess stenosis was 65.2%. -

Adult Spinal Deformity and Its Relationship with Height Loss
Shimizu et al. BMC Musculoskeletal Disorders (2020) 21:422 https://doi.org/10.1186/s12891-020-03464-2 RESEARCH ARTICLE Open Access Adult spinal deformity and its relationship with height loss: a 34-year longitudinal cohort study Mutsuya Shimizu1* , Tetsuya Kobayashi1, Hisashi Chiba2, Issei Senoo1, Hiroshi Ito1, Keisuke Matsukura3 and Senri Saito3 Abstract Background: Age-related height loss is a normal physical change that occurs in all individuals over 50 years of age. Although many epidemiological studies on height loss have been conducted worldwide, none have been long- term longitudinal epidemiological studies spanning over 30 years. This study was designed to investigate changes in adult spinal deformity and examine the relationship between adult spinal deformity and height loss. Methods: Fifty-three local healthy subjects (32 men, 21 women) from Furano, Hokkaido, Japan, volunteered for this longitudinal cohort study. Their heights were measured in 1983 and again in 2017. Spino-pelvic parameters were compared between measurements obtained in 1983 and 2017. Individuals with height loss were then divided into two groups, those with degenerative spondylosis and those with degenerative lumbar scoliosis, and different characteristics were compared between the two groups. Results: The mean age of the subjects was 44.4 (31–55) years at baseline and 78.6 (65–89) years at the final follow- up. The mean height was 157.4 cm at baseline and 153.6 cm at the final follow-up, with a mean height loss of 3.8 cm over 34.2 years. All parameters except for thoracic kyphosis were significantly different between measurements taken in 1983 and 2017 (p < 0.05).