Oliguria: Perioperative Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines for Management of Acute Renal Failure (Acute Kidney Injury)
Guidelines for management of Acute Renal Failure (Acute Kidney Injury) Children’s Kidney Centre University Hospital of Wales Cardiff CF14 4XW DISCLAIMER: These guidelines were produced in good faith by the author(s) reviewing available evidence/opinion. They were designed for use by paediatric nephrologists at the University Hospital of Wales, Cardiff for children under their care. They are neither policies nor protocols but are intended to serve only as guidelines. They are not intended to replace clinical judgment or dictate care of individual patients. Responsibility and decision-making (including checking drug doses) for a specific patient lie with the physician and staff caring for that particular patient. Version 1, S. Hegde/Feb 2009 Guidelines on management of Acute Renal Failure (Acute Kidney Injury) Definition of ARF (now referred to as AKI) • Acute renal failure is a sudden decline in glomerular filtration rate (usually marked by rise in serum creatinine & urea) which is potentially reversible with or without oliguria. • Oliguria defined as urine output <300ml/m²/day or < 0.5 ml/kg/h (<1 ml/kg/h in neonates). • Acute on chronic renal failure suggested by poor growth, history of polyuria and polydipsia, and evidence of renal osteodystrophy However, immediately after a kidney injury, serum creatinine & urea levels may be normal, and the only sign of a kidney injury may be decreased urine production. A rise in the creatinine level can result from medications (e.g., cimetidine, trimethoprim) that inhibit the kidney’s tubular secretion. A rise in the serum urea level can occur without renal injury, such as in GI or mucosal bleeding, steroid use, or protein loading. -

Current Current
CP_0406_Cases.final 3/17/06 2:57 PM Page 67 Current p SYCHIATRY CASES THAT TEST YOUR SKILLS Chronic enuresis has destroyed 12-year-old Jimmy’s emotional and social functioning. The challenge: restore his self-esteem by finding out why can’t he stop wetting his bed. The boy who longed for a ‘dry spell’ Tanvir Singh, MD Kristi Williams, MD Fellow, child® Dowdenpsychiatry ResidencyHealth training Media director, psychiatry Medical University of Ohio, Toledo CopyrightFor personal use only HISTORY ‘I CAN’T FACE MYSELF’ during regular checkups and refer to a psychia- immy, age 12, is referred to us by his pediatri- trist only if the child has an emotional problem J cian, who is concerned about his “frequent secondary to enuresis or a comorbid psychiatric nighttime accidents.” His parents report that he wets disorder. his bed 5 to 6 times weekly and has never stayed con- Once identified, enuresis requires a thorough sistently dry for more than a few days. assessment—including its emotional conse- The accidents occur only at night, his parents quences, which for Jimmy are significant. In its say. Numerous interventions have failed, including practice parameter for treating enuresis, the restricting fluids after dinner and awakening the boy American Academy of Child and Adolescent overnight to make him go to the bathroom. Psychiatry (AACAP)1 suggests that you: Jimmy, a sixth-grader, wonders if he will ever Take an extensive developmental and family stop wetting his bed. He refuses to go to summer history. Find out if the child was toilet trained and camp or stay overnight at a friend’s house, fearful started walking, talking, or running at an appro- that other kids will make fun of him after an acci- priate age. -

Urinary System Diseases and Disorders
URINARY SYSTEM DISEASES AND DISORDERS BERRYHILL & CASHION HS1 2017-2018 - CYSTITIS INFLAMMATION OF THE BLADDER CAUSE=PATHOGENS ENTERING THE URINARY MEATUS CYSTITIS • MORE COMMON IN FEMALES DUE TO SHORT URETHRA • SYMPTOMS=FREQUENT URINATION, HEMATURIA, LOWER BACK PAIN, BLADDER SPASM, FEVER • TREATMENT=ANTIBIOTICS, INCREASE FLUID INTAKE GLOMERULONEPHRITIS • AKA NEPHRITIS • INFLAMMATION OF THE GLOMERULUS • CAN BE ACUTE OR CHRONIC ACUTE GLOMERULONEPHRITIS • USUALLY FOLLOWS A STREPTOCOCCAL INFECTION LIKE STREP THROAT, SCARLET FEVER, RHEUMATIC FEVER • SYMPTOMS=CHILLS, FEVER, FATIGUE, EDEMA, OLIGURIA, HEMATURIA, ALBUMINURIA ACUTE GLOMERULONEPHRITIS • TREATMENT=REST, SALT RESTRICTION, MAINTAIN FLUID & ELECTROLYTE BALANCE, ANTIPYRETICS, DIURETICS, ANTIBIOTICS • WITH TREATMENT, KIDNEY FUNCTION IS USUALLY RESTORED, & PROGNOSIS IS GOOD CHRONIC GLOMERULONEPHRITIS • REPEATED CASES OF ACUTE NEPHRITIS CAN CAUSE CHRONIC NEPHRITIS • PROGRESSIVE, CAUSES SCARRING & SCLEROSING OF GLOMERULI • EARLY SYMPTOMS=HEMATURIA, ALBUMINURIA, HTN • WITH DISEASE PROGRESSION MORE GLOMERULI ARE DESTROYED CHRONIC GLOMERULONEPHRITIS • LATER SYMPTOMS=EDEMA, FATIGUE, ANEMIA, HTN, ANOREXIA, WEIGHT LOSS, CHF, PYURIA, RENAL FAILURE, DEATH • TREATMENT=LOW NA DIET, ANTIHYPERTENSIVE MEDS, MAINTAIN FLUIDS & ELECTROLYTES, HEMODIALYSIS, KIDNEY TRANSPLANT WHEN BOTH KIDNEYS ARE SEVERELY DAMAGED PYELONEPHRITIS • INFLAMMATION OF THE KIDNEY & RENAL PELVIS • CAUSE=PYOGENIC (PUS-FORMING) BACTERIA • SYMPTOMS=CHILLS, FEVER, BACK PAIN, FATIGUE, DYSURIA, HEMATURIA, PYURIA • TREATMENT=ANTIBIOTICS, -

Case Report Jun 2013; Vol 23 (No 3), Pp: 360-362
Iran J Pediatr Case Report Jun 2013; Vol 23 (No 3), Pp: 360-362 Spontaneous Rupture of Kidney Due to Posterior Urethral Valve– Diagnostic Difficulties Katarzyna Kiliś-Pstrusińska1 ,MD,PhD; Agnieszka Pukajło-Marczyk1,MD; Dariusz Patkowski2 ,MD,PhD; Urszula Zalewska-Dorobisz3 ,MD,PhD; Danuta Zwolińska1 ,MD,PhD 1. Department of Paediatric Nephrology, Wroclaw Medical University, Wrocław, Poland 2. Department of Paediatric Surgery and Urology, Wroclaw Medical University, Wrocław, Poland 3. Department of Radiology, Wroclaw Medical University, Wrocław, Poland Received: Jan 18, 2012; Accepted: Aug 04, 2012; First Online Available: Nov 22, 2012 Abstract Background: Spontaneous kidney rupture could develop in the course of posterior urethral valve (PUV), the most common cause of outflow urinary tract obstruction in male infants. However, urinary extravasation is a rare complication among this group of children. Case Presentation: Our case report presents diagnostic difficulties connected with spontaneous kidney rupture due to PUV in a 6 week-old infant. Due to not equivocal images, thundery course of disease and rapid deterioration in the infant`s condition, the patient required an urgent laparatomy. Conclusion: This case showed that the investigation of renal abnormalities during early neonatal period, is very important specifically in PUV that can lead to kidney rupture. Iranian Journal of Pediatrics, Volume 23 (Number 3), June 2013, Pages: 360-362 Key Words: Urinoma; Kidney Rupture; Urinary Extravasation; Posterior Urethral Valve Introduction rare complication among this group of children. The clinical manifestation is often nonspecific. Kidney rupture in developmental age most often Our report presents diagnostic difficulties takes place in the aftermath of multiorgan trauma, connected with spontaneous kidney rupture due especially when urinary abnormalities or to PUV in a 6-week old male. -

History & Physical Format
History & Physical Format SUBJECTIVE (History) Identification name, address, tel.#, DOB, informant, referring provider CC (chief complaint) list of symptoms & duration. reason for seeking care HPI (history of present illness) - PQRST Provocative/palliative - precipitating/relieving Quality/quantity - character Region - location/radiation Severity - constant/intermittent Timing - onset/frequency/duration PMH (past medical /surgical history) general health, weight loss, hepatitis, rheumatic fever, mono, flu, arthritis, Ca, gout, asthma/COPD, pneumonia, thyroid dx, blood dyscrasias, ASCVD, HTN, UTIs, DM, seizures, operations, injuries, PUD/GERD, hospitalizations, psych hx Allergies Meds (Rx & OTC) SH (social history) birthplace, residence, education, occupation, marital status, ETOH, smoking, drugs, etc., sexual activity - MEN, WOMEN or BOTH CAGE Review Ever Feel Need to CUT DOWN Ever Felt ANNOYED by criticism of drinking Ever Had GUILTY Feelings Ever Taken Morning EYE OPENER FH (family history) age & cause of death of relatives' family diseases (CAD, CA, DM, psych) SUBJECTIVE (Review of Systems) skin, hair, nails - lesions, rashes, pruritis, changes in moles; change in distribution; lymph nodes - enlargement, pain bones , joints muscles - fractures, pain, stiffness, weakness, atrophy blood - anemia, bruising head - H/A, trauma, vertigo, syncope, seizures, memory eyes- visual loss, diplopia, trauma, inflammation glasses ears - deafness, tinnitis, discharge, pain nose - discharge, obstruction, epistaxis mouth - sores, gingival bleeding, teeth, -

Signs and Symptoms of Urinary System Diseases
SIGNS AND SYMPTOMS OF URINARY SYSTEM DISEASES LECTURE IN INTERNAL MEDICINE PROPAEDEUTICS M. Yabluchansky, L. Bogun, L.Martymianova, O. Bychkova, N. Lysenko, N. Makienko, E. Tomina, E. Golubkina V.N. Karazin National University Medical School’ Internal Medicine Dept. http://kottke.org/tag/infoviz Plan of the lecture • The importance(value) of a human kidney • Reminder – how do kidneys work – the primary function – purpose • History-taking • Patient’s examination – clinical – laboratory – instrumental • Spectrum of urinary system diseases • Urinary system diseases’ symptoms and syndromes – symptoms – urinary syndrome – nephrotic syndrome – nephritic syndrome – urinary tract obstruction syndrome – hypertensive syndrome • Glossary of urinary pathology’ terms http://images.emedicinehealth.com/images/illustrations/urinary_structures.jpg The price of a human kidney The human kidney is the body’s filter. It cleans 180 liters of liquid per day, retaining the good stuff and expelling the bad. Most fortuitously, humans are born with two kidneys. If one of them becomes damaged, the other one can pick up the slack. If both your kidneys fail, however, your body will be filled with harmful toxins. Without medical intervention, such patients will die within several weeks Reminder: how kidneys work https://www.youtube.com/watch?v=aj-gbnOB4jM http://venturebeat.com/wp-content/uploads/2012/08/kidneys.jpg Reminder: the primary urinary system functions • maintain homeostasis • regulate fluids and electrolytes • eliminate waste products • maintain blood pressure (BP) • involved with red blood cell (RBC) production • involved with bone metabolism Reminder: purpose • General evaluation of health • Diagnosis of disease or disorders of the kidneys or urinary tract • Diagnosis of other systemic diseases that affect kidney function • Monitoring of patients with diabetes • Screening for drug toxicity (eg. -

URINARY TRACT DISORDERS in HORSES- Advances in Diagnostics and Treatments Thomas J
URINARY TRACT DISORDERS IN HORSES- Advances in Diagnostics and Treatments Thomas J. Divers, DVM, DACVIM, DAVECC College of Veterinary Medicine Cornell University, Ithaca, New York USA Introduction- Drs. Lisle George and Robert Whitlock in 1976 during my residency encouraged me to develop and interest in studying urinary tract diseases in large animals. I am grateful to him for encouraging me to do so. In these notes, I will give a brief overview of equine urinary tract disorders pointing out some of the discoveries or observations made over the last 45 years. Acute Renal failure/ Acute Kidney Injury In most of the recent literature the term acute renal failure (ARF) has been replaced by acute kidney injury (AKI). One commonly used definition of AKI is an acute increase in serum creatinine of 0.3 mg/dl or greater and oliguria. The switch in terms from ARF to AKI is semantic and in my mind has not improved our understanding or management of the disease. Uremia is the clinical condition caused by renal failure and accumulation of harmful products in the blood associated with decline in kidney function. AKI leading to renal failure and uremia can result from either intrinsic (kidney) disease/dysfunction or obstruction/rupture of the urinary tract. Acute renal failure is more common in large animals than chronic renal failure (CRF) and we can often reverse the disease process in ARF. Intrinsic causes of acute renal failure (ARF/AKI) include those disorders that cause significant functional decreases in glomerular filtration rate (GFR), mostly due to abnormal glomerular filtration pressures (common with septic shock), or morphologic nephron damage (common with nephrotoxins) or both. -

ACS/ASE Medical Student Core Curriculum Postoperative Care
ACS/ASE Medical Student Core Curriculum Postoperative Care POSTOPERATIVE CARE Prompt assessment and treatment of postoperative complications is critical for the comprehensive care of surgical patients. The goal of the postoperative assessment is to ensure proper healing as well as rule out the presence of complications, which can affect the patient from head to toe, including the neurologic, cardiovascular, pulmonary, renal, gastrointestinal, hematologic, endocrine and infectious systems. Several of the most common complications after surgery are discussed below, including their risk factors, presentation, as well as a practical guide to evaluation and treatment. Of note, fluids and electrolyte shifts are normal after surgery, and their management is very important for healing and progression. Please see the module on Fluids and Electrolytes for further discussion. Epidemiology/Pathophysiology I. Wound Complications Proper wound healing relies on sufficient oxygen delivery to the wound, lack of bacterial and necrotic contamination, and adequate nutritional status. Factors that can impair wound healing and lead to complications include bacterial infection (>106 CFUs/cm2), necrotic tissue, foreign bodies, diabetes, smoking, malignancy, malnutrition, poor blood supply, global hypotension, hypothermia, immunosuppression (including steroids), emergency surgery, ascites, severe cardiopulmonary disease, and intraoperative contamination. Of note, when reapproximating tissue during surgery, whether one is closing skin or performing a bowel anastomosis, tension on the wound edges is an important factor that contributes to proper healing. If there is too much tension on the wound, there will be local ischemia within the microcirculation, which will compromise healing. Common wound complications include infection, dehiscence, and incisional hernia. Wound infections, or surgical site infections (SSI), can occur in the surgical field from deep organ spaces to superficial skin and are due to bacterial contamination. -

Abnormalities of Urine
Appendix A Abnormalities of urine Urine testing by the use of observation and dipstick tests is a procedure carried out on an almost daily basis by nursing staff. Since this procedure is frequently used as a primary screening technique it is important that nurses have a knowledge of the meaning of the test results and what constitute abnormal readings. Correct interpretation and reporting of the results may not only lead to an earlier diagnosis and hence treat ment for the patient but also to a financial saving, since it may obviate the need for further analysis of the urine in the laboratory. Observation of the urine is a comparatively straight forward but nonetheless important activity. The exact nature and the range of substances which can be detected using test strips vary from manufacturer to manufacturer and this section will give an outline of the substances most commonly tested for, and how their presence in urine may be interpreted. It is important to stress that accurate testing using this method relies on following the manufacturers' instructions particularly in relation to reading the results at specific times which may be necessary for quantitative analysis . Fresh urine should always be used for the test. OBSERVATION OF THE URINE Colour The colour of normal urine varies between a very light yellow to a dark amber. Normally this is dependent on the concen tration of the urine and diet. Certain diseases mayaiso have an effect on this. Consistently light coloured urine may be the result of excessive urine production, as would occur in diabetes mellitus and diabetes insipidus, whereas production of very Observation of the urine 161 dark urine may be indicative of some form of hepatic or biliary disease and the presence of large amounts of urobilinogen. -
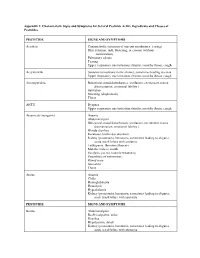
Appendix 2: Characteristic Signs and Symptoms for Several Pesticide Active Ingredients and Classes of Pesticides
Appendix 2: Characteristic Signs and Symptoms for Several Pesticide Active Ingredients and Classes of Pesticides PESTICIDE SIGNS AND SYMPTOMS Acrolein Conjunctivitis (irritation of mucous membranes, tearing) Skin irritation, rash, blistering, or erosion (without sensitization) Pulmonary edema Tearing Upper respiratory tract irritation: rhinitis, scratchy throat, cough Acrylonitrile Seizures/convulsions (tonic-clonic), sometimes leading to coma Upper respiratory tract irritation: rhinitis, scratchy throat, cough Aminopyridine Behavioral-mood disturbances (confusion, excitement, mania, disorientation, emotional lability ) Salivation Sweating (diaphoresis) Thirst ANTU Dyspnea Upper respiratory tract irritation: rhinitis, scratchy throat, cough Arsenicals (inorganic) Anemia Abdominal pain Behavioral-mood disturbances (confusion, excitement, mania, disorientation, emotional lability ) Bloody diarrhea Keratoses, brown discoloration Kidney (proteinuria, hematuria, sometimes leading to oliguria, acute renal failure with azotemia Leukopenia, thrombocytopenia Metallic taste in mouth Paralysis, paresis (muscle weakness) Paresthesia of extremities Runny nose Stomatitis Thirst Arsine Anemia Chills Hemoglobinuria Hemolysis Hyperkalemia Kidney (proteinuria, hematuria, sometimes leading to oliguria, acute renal failure with azotemia PESTICIDE SIGNS AND SYMPTOMS Borate Abdominal pain Beefy red palms, soles Diarrhea Hypotension, shock Kidney (proteinuria, hematuria, sometimes leading to oliguria, acute renal failure with azotemia Nervous system depression -

Department of Neurology
Department of Neurology ® Follow Up FIRST NAME: LAST NAME: DATE OF BIRTH: PRIMARY CARE PROVIDER & CLINIC: ® PREVIOUS NEUROLOGIST: REVIEW OF SYSTEMS: (please mark all that apply) 1. Head, Eyes, Ears, Nose, Throat, Lymph Nodes: — Headaches — Neck swelling — Glaucoma — Double vision — Pain and/or drainage from ears — Teethe grinding / clenching — Hoarseness of voice — Nasal and/or sinus congestion — Headaches — Tinnitus (buzzing or humming) — Visual loss or change — Hearing problems — Photophobia (light bothers eyes) — Nose bleeds — Vision problems — Swollen and/or painful lymph nodes — Neck stiffness — Dental problems — Head trauma — Sneezing — Sinus problems — Deafness — Sore throat 2. Respiratory System: — Shortness of breath — Cough — Sputum/secretion production — Hemoptysis — Wheezing — Breathing difficulty 3. Cardiovascular System: — Chest pain, discomfort, heaviness, tightness — Orthopnea (sleeping on two or more pillows) — Palpitations — Shortness of breath with exertion — Leg swelling — Chest pain — PND (waking up short of breath) — High blood pressure — Heartburn 1 of 2 Please complete all pages Neurology Locations The Portland Clinic - South The Portland Clinic - Downtown THEPORTLANDCLINIC.COM [11009 8/14] 6640 SW Redwood Lane 800 SW 13th Ave Portland, OR 97224 Portland, OR 97205 (503) 620-7358 (503) 221-0161 4. Gastrointestinal System: — Anorexia (poor appetite) — Hematochezia (red blood in bowel movements) — Dysphagia (difficulty swollowing) — Nausea and/or vomiting — Melena (black bowel movements) — Stomach pain — Constipation or diarrhea — Jaundice — Weight change: loss ____ lbs , gain____lbs — Weight loss or gain — Abdominal pain 5. Genitourinary System: — Hematuria — Nocturia (urination at night) — Symptoms of menopause — Oliguria (infrequent urination) — Frequency (frequent urination) — Irregular periods — Incontinence — Pyuria (cloudy urine) — PMS — Heavy menstrual flow — Urgency (sensation to urinate) — Bladder problems — Polyuria (urination of large volumes of urnie) — Sexual dysfunction — Excessive urination or thirst 6. -

Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 Published: November 27, 2017
Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 Published: November 27, 2017 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Common Terminology Criteria for Adverse Events (CTCAE) v5.0 Publish Date: November 27, 2017 Introduction Grades Grade 5 The NCI Common Terminology Criteria for Grade refers to the severity of the AE. The CTCAE Grade 5 (Death) is not appropriate for some AEs Adverse Events is a descriptive terminology which displays Grades 1 through 5 with unique clinical and therefore is not an option. can be utilized for Adverse Event (AE) reporting. A descriptions of severity for each AE based on this grading (severity) scale is provided for each AE general guideline: Definitions term. A brief Definition is provided to clarify the Grade 1 Mild; asymptomatic or mild meaning of each AE term. A single dash (-) symptoms; clinical or diagnostic indicates a Definition is not available. SOC observations only; intervention System Organ Class (SOC), the highest level of the not indicated. 1 MedDRA hierarchy, is identified by anatomical or Grade 2 Moderate; minimal, local or Navigational Notes physiological system, etiology, or purpose (e.g., noninvasive intervention A Navigational Note is used to assist the reporter SOC Investigations for laboratory test results). indicated; limiting age- in choosing a correct AE. It may list other AEs that CTCAE terms are grouped by MedDRA Primary appropriate instrumental ADL*. should be considered in addition to or in place of SOCs. Within each SOC, AEs are listed and Grade 3 Severe or medically significant but not the AE in question. A single dash (-) indicates a accompanied by descriptions of severity (Grade).