Downloaded Via Formed by Means of Phylogeny.Fr (
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Optimization of Agro-Residues As Substrates for Pleurotus
Wu et al. AMB Expr (2019) 9:184 https://doi.org/10.1186/s13568-019-0907-1 ORIGINAL ARTICLE Open Access Optimization of agro-residues as substrates for Pleurotus pulmonarius production Nan Wu1†, Fenghua Tian1†, Odeshnee Moodley1, Bing Song1, Chuanwen Jia1, Jianqiang Ye2, Ruina Lv1, Zhi Qin3 and Changtian Li1* Abstract The “replacing wood by grass” project can partially resolve the confict between mushroom production and balancing the ecosystem, while promoting agricultural economic sustainability. Pleurotus pulmonarius is an economically impor- tant edible and medicinal mushroom, which is traditionally produced using a substrate consisting of sawdust and cottonseed hulls, supplemented with wheat bran. A simplex lattice design was applied to systemically optimize the cultivation of P. pulmonarius using agro-residues as the main substrate to replace sawdust and cottonseed hulls. The efects of difering amounts of wheat straw, corn straw, and soybean straw on the variables of yield, mycelial growth rate, stipe length, pileus length, pileus width, and time to harvest were demonstrated. Results indicated that a mix of wheat straw, corn straw, and soybean straw may have signifcantly positive efects on each of these variables. The high yield comprehensive formula was then optimized to include 40.4% wheat straw, 20.3% corn straw, 18.3% soybean straw, combined with 20.0% wheat bran, and 1.0% light CaCO3 (C/N 42.50). The biological efciency was 15.2% greater than that of the control. Most encouraging was the indication= that the high yield comprehensive formula may shorten the time to reach the reproductive stage by 6 days, compared with the control. -

The Biosynthesis of Plant and Fungal Sesquiterpenoids in Ustilago Maydis and Discovery of a Bioactive Compound from Fistulina Hepatica
The biosynthesis of plant and fungal sesquiterpenoids in Ustilago maydis and discovery of a bioactive compound from Fistulina hepatica Inaugural-Dissertation Zur Erlangung des Doktorgrades der Mathematisch-Naturwissenschaftlichen Fakultät der Heinrich-Heine-Universität Düsseldorf vorgelegt von Jungho Lee aus Daegu Düsseldorf, September 2020 aus dem Institut für Mikrobiologie der Heinrich-Heine-Universität Düsseldorf Gedruckt mit der Genehmigung der Mathematisch-Naturwissenschaftlichen Fakultät der Heinrich-Heine-Universität Düsseldorf Referent: Prof. Dr. Michael Feldbrügge Korreferent : Prof. Dr. Julia Frunzke Tag der mündlichen Prüfung: 26. Oktober 2020 Eidesstattliche Erklärung Ich versichere an Eides Statt, dass die Dissertation von mir selbständig und ohne unzulässige fremde Hilfe unter Beachtung der „Grundsätze zur Sicherung guter wissenschaftlicher Praxis an der Heinrich-Heine-Universität Düsseldorf“ erstellt worden ist. Die Dissertation wurde in ihrer jetzigen oder einer ähnlichen Form noch bei keiner anderen Hochschule eingereicht. Ich habe zuvor keine erfolglosen Promotionsversuche unternommen. Ort, Datum Unterschrift Die Untersuchungen zur vorliegenden Arbeit wurden von Oktober 2016 bis September 2020 in Düsseldorf an der Heinrich-Heine-Universität in dem Institut für Mikrobiologie unter der Betreuung von Herrn Prof. Dr. Michael Feldbrügge durchgeführt. Teile dieser Arbeit wurden veröffentlicht in: Lee, J., Hilgers, F., Loeschke, A., Jaeger, K. E., Feldbrügge, M., 2020, Ustilago maydis serves as a novel production host for the synthesis of plant and fungal sesquiterpenoids. Frontiers in Microbiology 11, 1655. Lee, J., Shi, Y., Grün, P., Gube, M., Feldbrügge, M., Bode, H. B., Hennicke, F., 2020, Identification of feldin, an antifungal polyine from the beefsteak fungus Fistulina hepatica. Biomolecules 10, 1502. Summary Summary Sesquiterpenoids are important secondary metabolites with various pharma- and nutraceutical properties. -

Oyster Mushroom) Related with Its Chemical Composition: a Review on the Past Decade Findings
Biotechnological, nutritional and therapeutic uses of Pleurotus spp. (Oyster mushroom) related with its chemical composition: A review on the past decade findings Rúbia Carvalho Gomes Corrêaa,b,c, Tatiane Brugnaric, Adelar Brachtc, Rosane Marina Peraltac, Isabel C.F.R. Ferreiraa,* aMountain Research Centre (CIMO), ESA, Polytechnic Institute of Bragança, Campus de Santa Apolónia, 1172, 5301-855 Bragança, Portugal. bCAPES Foundation, Ministry of Education of Brazil, 70.040-020, Brasília, DF, Brazil. cState University of Maringá, Department of Biochemistry, 87020-900, Maringá, PR, Brazil. * Author to whom correspondence should be addressed (Isabel C.F.R. Ferreira; e-mail: [email protected]; telephone +351-273-303219; fax +351-273-325405). 1 Abstract Background: The particular characteristics of growth and development of mushrooms in nature result in the accumulation of a variety of secondary metabolites, several of them with biological activities. The genus Pleurotus is a cosmopolitan group of mushrooms with high nutritional value and therapeutic properties, besides a wide array of biotechnological and environmental applications. Scope and approach: The present report aims to provide a critical review on aspects related to chemical compounds isolated from the genus Pleurotus with possible biotechnological, nutritional and therapeutic uses. Investigations on the genus have immensely accelerated during the last ten years, so that only reports published after 2005 have been considered. Key findings and conclusions: The most important Pleurotus species cultivated in large scale are P. ostreatus and P. pulmonarius. However, more than 200 species have already been investigated to various degrees. Both basidiomata and mycelia of Pleurotus are a great renewable and easily accessible source of functional foods/nutraceuticals and pharmaceuticals with antioxidant, antimicrobial, anti- inflammatory, antitumor and immunomodulatory effects. -

Transcriptional and Enzymatic Profiling of Pleurotus Ostreatus
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Academica-e Transcriptional and Enzymatic Profiling of Pleurotus ostreatus Laccase Genes in Submerged and Solid-State Fermentation Cultures Raúl Castanera,a Gúmer Pérez,a Alejandra Omarini,a Manuel Alfaro,a Antonio G. Pisabarro,a Vincenza Faraco,b,c Antonella Amore,b and Lucía Ramíreza Genetics and Microbiology Research Group, Department of Agrarian Production, Public University of Navarre, Pamplona, Spaina; Department of Organic Chemistry and Biochemistry, Complesso Universitario Monte S. Angelo, University of Naples Federico II, Naples, Italyb; and School of Biotechnological Sciences, University of Naples Federico II, Naples, Italyc The genome of the white rot basidiomycete Pleurotus ostreatus includes 12 phenol oxidase (laccase) genes. In this study, we ex- amined their expression profiles in different fungal strains under different culture conditions (submerged and solid cultures) Downloaded from and in the presence of a wheat straw extract, which was used as an inducer of the laccase gene family. We used a reverse tran- scription-quantitative PCR (RT-qPCR)-based approach and focused on determining the reaction parameters (in particular, the reference gene set for the normalization and reaction efficiency determinations) used to achieve an accurate estimation of the relative gene expression values. The results suggested that (i) laccase gene transcription is upregulated in the induced submerged fermentation (iSmF) cultures but downregulated in the solid fermentation (SSF) cultures, (ii) the Lacc2 and Lacc10 genes are the main sources of laccase activity in the iSmF cultures upon induction with water-soluble wheat straw extracts, and (iii) an addi- tional, as-yet-uncharacterized activity (Unk1) is specifically induced in SSF cultures that complements the activity of Lacc2 and Lacc10. -

Korrdel1lic Avhandl Ahmad Zayny
Faculty of Pharmacy, Uppsala University LICENTIATE THESES 50 Vitamin D metabolism in osteoblast-like cells: effects of drugs on inactivation by CYP24A1 by Ahmad Zayny 2018 Faculty of Pharmacy, Uppsala University LICENTIATE THESES 50 Summary Vitamin D metabolism in osteoblast-like cells: effects of drugs on inactivation by CYP24A1 by Ahmad Zayny 2018 2 This licentiate thesis will be defended on Wednesday, May 30, 2018 at 13:15 in C4:305, BMC, Husargatan 3, Uppsala. The presentation and discussion will be conducted in Swedish. Abstract Zayny, A. 2018 Vitamin D metabolism in osteoblast-like cells: effects of drugs on inactivation by CYP24A1 Vitamin D is essential for bone function, and deficiency in active vitamin D hormone can lead to bone disorders. Long-term treatment with glucocorticoids and antiretroviral drugs used to treat HIV infection, results in osteoporosis and increased risk of fractures. Much remains unclear regarding the effects of these compounds in bone cells. In the current study, human osteosarcoma Saos-2 cells and primary human osteoblasts were found to express mRNA for the vitamin D receptor as well as activating and deactivating enzymes in vitamin D3 metabolism. These bone cells exhibited CYP24A1-mediated 24-hydroxylation, involved in deactivation of the active vitamin form. However, bioactivating vitamin D3 hydroxylase activities were not detected in either of these cells, indicating that local vitamin D bioactivation is not significant in osteoblasts. Several glucocorticoids and antiretroviral drugs, including prednisolone, efavirenz and ritonavir, down regulated CYP24A1 mRNA expression. Prednisolone and ritonavir also down regulated CYP24A1-mediated 24-hydroxylase activity in both Saos-2 and primary human osteoblasts. -

Agarics-Stature-Types.Pdf
Gilled Mushroom Genera of Chicago Region, by stature type and spore print color. Patrick Leacock – June 2016 Pale spores = white, buff, cream, pale green to Pinkish spores Brown spores = orange, Dark spores = dark olive, pale lilac, pale pink, yellow to pale = salmon, yellowish brown, rust purplish brown, orange pinkish brown brown, cinnamon, clay chocolate brown, Stature Type brown smoky, black Amanitoid Amanita [Agaricus] Vaginatoid Amanita Volvariella, [Agaricus, Coprinus+] Volvopluteus Lepiotoid Amanita, Lepiota+, Limacella Agaricus, Coprinus+ Pluteotoid [Amanita, Lepiota+] Limacella Pluteus, Bolbitius [Agaricus], Coprinus+ [Volvariella] Armillarioid [Amanita], Armillaria, Hygrophorus, Limacella, Agrocybe, Cortinarius, Coprinus+, Hypholoma, Neolentinus, Pleurotus, Tricholoma Cyclocybe, Gymnopilus Lacrymaria, Stropharia Hebeloma, Hemipholiota, Hemistropharia, Inocybe, Pholiota Tricholomatoid Clitocybe, Hygrophorus, Laccaria, Lactarius, Entoloma Cortinarius, Hebeloma, Lyophyllum, Megacollybia, Melanoleuca, Inocybe, Pholiota Russula, Tricholoma, Tricholomopsis Naucorioid Clitocybe, Hygrophorus, Hypsizygus, Laccaria, Entoloma Agrocybe, Cortinarius, Hypholoma Lactarius, Rhodocollybia, Rugosomyces, Hebeloma, Gymnopilus, Russula, Tricholoma Pholiota, Simocybe Clitocyboid Ampulloclitocybe, Armillaria, Cantharellus, Clitopilus Paxillus, [Pholiota], Clitocybe, Hygrophoropsis, Hygrophorus, Phylloporus, Tapinella Laccaria, Lactarius, Lactifluus, Lentinus, Leucopaxillus, Lyophyllum, Omphalotus, Panus, Russula Galerinoid Galerina, Pholiotina, Coprinus+, -

And Dioxygenase Chemistries in a Single Active Site Promotes Heme Degradation
Unique coupling of mono- and dioxygenase chemistries in a single active site promotes heme degradation Toshitaka Matsuia,1, Shusuke Nambua, Celia W. Gouldingb,c, Satoshi Takahashia, Hiroshi Fujiid, and Masao Ikeda-Saitoa,1 aInstitute of Multidisciplinary Research for Advanced Materials, Tohoku University, Katahira, Aoba, Sendai 980-8577, Japan; bDepartment of Molecular Biology and Biochemistry, University of California, Irvine, CA 92697; cDepartment of Pharmaceutical Sciences, University of California, Irvine, CA 92697; and dDepartment of Chemistry, Graduate School of Humanities and Sciences, Nara Women’s University, Kitauoyanishi, Nara 630-8506, Japan Edited by Harry B. Gray, California Institute of Technology, Pasadena, CA, and approved February 26, 2016 (received for review November 26, 2015) − + Bacterial pathogens must acquire host iron for survival and colo- RH + O2 + 2e + 2H → ROH + H2O, [1] nization. Because free iron is restricted in the host, numerous pathogens have evolved to overcome this limitation by using a R + O → RðOÞ . [2] family of monooxygenases that mediate the oxidative cleavage of 2 2 heme into biliverdin, carbon monoxide, and iron. However, the Biological heme degradation proceeds through a unique self- etiological agent of tuberculosis, Mycobacterium tuberculosis, ac- oxidation mechanism where the substrate heme activates O complishes this task without generating carbon monoxide, which 2 potentially induces its latent state. Here we show that this unusual molecules. The canonical enzyme, heme oxygenase (HO), de- heme degradation reaction proceeds through sequential mono- grades heme into ferrous iron, carbon monoxide (CO), and bil- and dioxygenation events within the single active center of MhuD, iverdin by three successive monooxygenation reactions (Fig. 1A) a mechanism unparalleled in enzyme catalysis. -
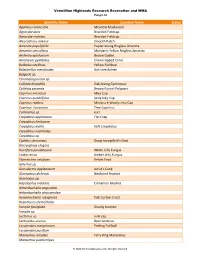
Scientific Name Common Name Status Agaricus Campestris
Vermillion Highlands Research Recreation and WMA Fungi List Scientific Name Common Name Status Agaricus campestris Meadow Mushroom Agrocybe dura Bearded Fieldcap Agrocybe molesta Bearded Fieldcap Aleurodiscus oakesii Smooth Patch Amanita populiphila Poplar-loving Ringless Amanita Amanita sinicoflava Mandarin Yellow Ringless Amanita Arrhenia epichysium Brown Goblet Artomyces pyxidatus Crown-tipped Coral Bolbitius vitellinus Yellow Fieldcap Boletinellus merulioides Ash-tree Bolete Bulgaria sp. Chromelosporium sp. Collybia dryophila Oak-loving Gymnopus Coltricia perennis Brown Funnel Polypore Coprinus micaceus Mica Cap Coprinus quadrifidus Scaly Inky Cap Coprinus radians Miniature Woolly Inky Cap Coprinus truncorum Tree Coprinus Cortinarius sp. cort Crepidotus applanatus Flat Crep Crepidotus herbarum Crepidotus mollis Soft Crepidotus Crepidotus nephrodes Crepidotus sp. Cyathus stercoreus Dung-loving Bird’s Nest Dacryopinax elegans Ductifera pululahuana White Jelly Fungus Exidia recisa Amber Jelly Fungus Flammulina velutipes Velvet Foot Galerina sp. Ganoderma applanatum Artist’s Conk Gloeoporus dichrous Bicolored Bracket Gymnopus sp. Hapalopilus nidulans Cinnamon Bracket Hohenbuehelia angustata Hohenbuehelia atrocaerulea Hymenochaete rubiginosa Oak Curtain Crust Hypomyces tremellicola Inocybe fastigiata Deadly Inocybe Inocybe sp. Lactarius sp. milk cap Lentinellus ursinus Bear Lentinus Lycoperdon marginatum Peeling Puffball Lycoperdon pusillum Marasmius oreades Fairy Ring Marasmius Marasmius pulcherripes © 2020 MinnesotaSeasons.com. All rights -

Wood Chip Fungi: Agrocybe Putaminum in the San Francisco Bay Area
Wood Chip Fungi: Agrocybe putaminum in the San Francisco Bay Area Else C. Vellinga Department of Plant and Microbial Biology, 111 Koshland Hall, Berkeley CA 94720-3102 [email protected] Abstract Agrocybe putaminum was found growing on wood chips in central coastal California; this appears to be the first record for North America. A short description of the species is given. Its habitat plus the characteristics of wood chip denizens are discussed. Wood chips are the fast food of the fungal world. The desir- able wood is exposed, there is a lot of it, and often the supply is replenished regularly. It is an especially good habitat for mush- room species that like it hot because a thick layer of wood chips is warmed relative to the surrounding environment by the activity of bacteria and microscopic fungi (Brown, 2003; Van den Berg and Vellinga, 1998). Thirty years ago wood chips were a rarity, but nowadays they are widely used in landscaping and gardening. A good layer of chips prevents weeds from germinating and taking over, which means less maintenance and lower costs. Chips also diminish evaporation and keep moisture in the soil. Trees and shrubs are often shredded and dumped locally, but there is also long-dis- tance transport of these little tidbits. Barges full of wood mulch cruise the Mississippi River, and trucks carry the mulch from city to city. This fast food sustains a steady stream of wood chip fungi that, as soon as they are established, fruit in large flushes and are suddenly everywhere. The fungi behave a bit like morels after a Figure 1. -

A Preliminary Checklist of Arizona Macrofungi
A PRELIMINARY CHECKLIST OF ARIZONA MACROFUNGI Scott T. Bates School of Life Sciences Arizona State University PO Box 874601 Tempe, AZ 85287-4601 ABSTRACT A checklist of 1290 species of nonlichenized ascomycetaceous, basidiomycetaceous, and zygomycetaceous macrofungi is presented for the state of Arizona. The checklist was compiled from records of Arizona fungi in scientific publications or herbarium databases. Additional records were obtained from a physical search of herbarium specimens in the University of Arizona’s Robert L. Gilbertson Mycological Herbarium and of the author’s personal herbarium. This publication represents the first comprehensive checklist of macrofungi for Arizona. In all probability, the checklist is far from complete as new species await discovery and some of the species listed are in need of taxonomic revision. The data presented here serve as a baseline for future studies related to fungal biodiversity in Arizona and can contribute to state or national inventories of biota. INTRODUCTION Arizona is a state noted for the diversity of its biotic communities (Brown 1994). Boreal forests found at high altitudes, the ‘Sky Islands’ prevalent in the southern parts of the state, and ponderosa pine (Pinus ponderosa P.& C. Lawson) forests that are widespread in Arizona, all provide rich habitats that sustain numerous species of macrofungi. Even xeric biomes, such as desertscrub and semidesert- grasslands, support a unique mycota, which include rare species such as Itajahya galericulata A. Møller (Long & Stouffer 1943b, Fig. 2c). Although checklists for some groups of fungi present in the state have been published previously (e.g., Gilbertson & Budington 1970, Gilbertson et al. 1974, Gilbertson & Bigelow 1998, Fogel & States 2002), this checklist represents the first comprehensive listing of all macrofungi in the kingdom Eumycota (Fungi) that are known from Arizona. -

And Stalpers (1978). Preparations For
PERSOONIA Published by the Rijksherbarium, Leiden Volume 13, Part 4, pp. 495-504 (1988) Auriculariopsis and the Schizophyllales J.A. Stalpers Centraalbureau voor Schimmelcultures, Baam* The cultural characters and the developmentofthe basidiomes of Auriculariopsis ampla (Lév.) Maire are described and compared with those of Schizophyllum commune Fr.: Fr. It is concluded that Auriculariopsis is very close to Schizo- phyllum and that the existence of the order Schizophyllales is not justified. Maire rather but Auriculariopsis ampla (Lev.) R. is a rare species in Europe, locally it be for the southern coastal sand dunes of the can quite common, example in more Netherlands. Its substrate is generally twigs of Populus, but occasionally Salix; there is one report from Rubus (Donk, 1959). In the older literature this species occurs almost uniformly under the name Cytidia flocculenta (Fr.) Hohn. & Litsch. Donk (1959) doubtedthat Thelephora flocculenta Fr. really was this species and Eriksson & Ryvarden (1975) found that authentic material contained Cylindrobasidium evolvens (Fr.: Fr.) Jiilich. METHODS Petri dishes on neutralized 2% malt and Isolates were grown in plastic agar (MEA) decoction at room 20° diffuse cherry agar (ChA) temperature (18— C) in daylight. Drop tests on laccase and tyrosinase were performed as describedby Kaarik (1965) and Stalpers (1978). Preparations for scanning electron microscopy were made according to Samson et al. (1979). CULTURAL CHARACTERS OF AURICULARIOPSIS AMPLA Growth on MEA rather fast, reaching 25—35 mm radius in 2 weeks, on ChA up to 40 mm. Odour insignificant. Advancing zone appressed to submerged, with irregularly undu- from lating outline; hyphae dense. Mycelial mat 1—2 mm the margin, cottony-woolly to the After four floccose, white. -

Re-Thinking the Classification of Corticioid Fungi
mycological research 111 (2007) 1040–1063 journal homepage: www.elsevier.com/locate/mycres Re-thinking the classification of corticioid fungi Karl-Henrik LARSSON Go¨teborg University, Department of Plant and Environmental Sciences, Box 461, SE 405 30 Go¨teborg, Sweden article info abstract Article history: Corticioid fungi are basidiomycetes with effused basidiomata, a smooth, merulioid or Received 30 November 2005 hydnoid hymenophore, and holobasidia. These fungi used to be classified as a single Received in revised form family, Corticiaceae, but molecular phylogenetic analyses have shown that corticioid fungi 29 June 2007 are distributed among all major clades within Agaricomycetes. There is a relative consensus Accepted 7 August 2007 concerning the higher order classification of basidiomycetes down to order. This paper Published online 16 August 2007 presents a phylogenetic classification for corticioid fungi at the family level. Fifty putative Corresponding Editor: families were identified from published phylogenies and preliminary analyses of unpub- Scott LaGreca lished sequence data. A dataset with 178 terminal taxa was compiled and subjected to phy- logenetic analyses using MP and Bayesian inference. From the analyses, 41 strongly Keywords: supported and three unsupported clades were identified. These clades are treated as fam- Agaricomycetes ilies in a Linnean hierarchical classification and each family is briefly described. Three ad- Basidiomycota ditional families not covered by the phylogenetic analyses are also included in the Molecular systematics classification. All accepted corticioid genera are either referred to one of the families or Phylogeny listed as incertae sedis. Taxonomy ª 2007 The British Mycological Society. Published by Elsevier Ltd. All rights reserved. Introduction develop a downward-facing basidioma.