On the Distribution and Conservation of Two ``Lost World'' Tepui Summit
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Catalogue of the Amphibians of Venezuela: Illustrated and Annotated Species List, Distribution, and Conservation 1,2César L
Mannophryne vulcano, Male carrying tadpoles. El Ávila (Parque Nacional Guairarepano), Distrito Federal. Photo: Jose Vieira. We want to dedicate this work to some outstanding individuals who encouraged us, directly or indirectly, and are no longer with us. They were colleagues and close friends, and their friendship will remain for years to come. César Molina Rodríguez (1960–2015) Erik Arrieta Márquez (1978–2008) Jose Ayarzagüena Sanz (1952–2011) Saúl Gutiérrez Eljuri (1960–2012) Juan Rivero (1923–2014) Luis Scott (1948–2011) Marco Natera Mumaw (1972–2010) Official journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(1) [Special Section]: 1–198 (e180). Catalogue of the amphibians of Venezuela: Illustrated and annotated species list, distribution, and conservation 1,2César L. Barrio-Amorós, 3,4Fernando J. M. Rojas-Runjaic, and 5J. Celsa Señaris 1Fundación AndígenA, Apartado Postal 210, Mérida, VENEZUELA 2Current address: Doc Frog Expeditions, Uvita de Osa, COSTA RICA 3Fundación La Salle de Ciencias Naturales, Museo de Historia Natural La Salle, Apartado Postal 1930, Caracas 1010-A, VENEZUELA 4Current address: Pontifícia Universidade Católica do Río Grande do Sul (PUCRS), Laboratório de Sistemática de Vertebrados, Av. Ipiranga 6681, Porto Alegre, RS 90619–900, BRAZIL 5Instituto Venezolano de Investigaciones Científicas, Altos de Pipe, apartado 20632, Caracas 1020, VENEZUELA Abstract.—Presented is an annotated checklist of the amphibians of Venezuela, current as of December 2018. The last comprehensive list (Barrio-Amorós 2009c) included a total of 333 species, while the current catalogue lists 387 species (370 anurans, 10 caecilians, and seven salamanders), including 28 species not yet described or properly identified. Fifty species and four genera are added to the previous list, 25 species are deleted, and 47 experienced nomenclatural changes. -

Amazon Alive: a Decade of Discoveries 1999-2009
Amazon Alive! A decade of discovery 1999-2009 The Amazon is the planet’s largest rainforest and river basin. It supports countless thousands of species, as well as 30 million people. © Brent Stirton / Getty Images / WWF-UK © Brent Stirton / Getty Images The Amazon is the largest rainforest on Earth. It’s famed for its unrivalled biological diversity, with wildlife that includes jaguars, river dolphins, manatees, giant otters, capybaras, harpy eagles, anacondas and piranhas. The many unique habitats in this globally significant region conceal a wealth of hidden species, which scientists continue to discover at an incredible rate. Between 1999 and 2009, at least 1,200 new species of plants and vertebrates have been discovered in the Amazon biome (see page 6 for a map showing the extent of the region that this spans). The new species include 637 plants, 257 fish, 216 amphibians, 55 reptiles, 16 birds and 39 mammals. In addition, thousands of new invertebrate species have been uncovered. Owing to the sheer number of the latter, these are not covered in detail by this report. This report has tried to be comprehensive in its listing of new plants and vertebrates described from the Amazon biome in the last decade. But for the largest groups of life on Earth, such as invertebrates, such lists do not exist – so the number of new species presented here is no doubt an underestimate. Cover image: Ranitomeya benedicta, new poison frog species © Evan Twomey amazon alive! i a decade of discovery 1999-2009 1 Ahmed Djoghlaf, Executive Secretary, Foreword Convention on Biological Diversity The vital importance of the Amazon rainforest is very basic work on the natural history of the well known. -

Zootaxa, Herpetological Results of the 2002 Expedition to Sarisarinama, A
ZOOTAXA 1942 Herpetological results of the 2002 expedition to Sarisariñama, a tepui in Venezuelan Guayana, with the description of five new species CESAR L. BARRIO-AMOROS & CHARLES BREWER-CARIAS Magnolia Press Auckland, New Zealand Cesar L. Barrio-Amoros & Charles Brewer-Carias Herpetological results of the 2002 expedition to Sarisariñama, a tepui in Venezuelan Guayana, with the description of five new species (Zootaxa 1942) 68 pp.; 30 cm. 26 Nov. 2008 ISBN 978-1-86977-269-7 (paperback) ISBN 978-1-86977-270-3 (Online edition) FIRST PUBLISHED IN 2008 BY Magnolia Press P.O. Box 41-383 Auckland 1346 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2008 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. This authorization does not extend to any other kind of copying, by any means, in any form, and for any purpose other than private research use. ISSN 1175-5326 (Print edition) ISSN 1175-5334 (Online edition) 2 · Zootaxa 1942 © 2008 Magnolia Press BARRIO-AMORÓS & BREWER-CARÍAS Zootaxa 1942: 1–68 (2008) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2008 · Magnolia Press ISSN 1175-5334 (online edition) Herpetological results of the 2002 expedition to Sarisariñama, a tepui in Venezuelan Guayana, with the description of five new species CÉSAR L. BARRIO-AMORÓS1 & CHARLES BREWER-CARÍAS2 1Fundación AndígenA, Apartado Postal 210, 5101-A Mérida, Venezuela. -
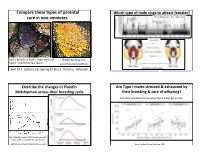
Compare These Types of Parental Care in Non-‐Amniotes
5/4/16 Compare these types of parental Which type of male sings to a6ract females? care in non-‐amniotes. McIver, Marchaterre,. Rice, & Bass, 2014 Time (sec) Type I: 80-‐90% of males, large, territorial Plainfin Midshipman Type II: small body, fast sperm hOp://www.basslab.org/photos/ hOp://bio3520.nicerweb.com/Locked/chap/ch03/sonic_muscles.html Biol 417, Lecture 16, Spring 2016, Dr. Karen E. Petersen hOp://blogs.scienQficamerican.com/brainwaves/what-‐singing-‐fish-‐reveal-‐about-‐speech-‐and-‐hearing/ hOp://www.livescience.com/27237-‐fish-‐sings-‐for-‐mates.html Describe the changes in Plainfin Are Type I males stressed & exhausted by Midshipman across their breeding cycle their breeding & care of offspring? ScienQsts wanted to know when these males get to eat…. (A) CollecQon depth & (B) GonadosomaQc index (GSI) of females & type I males Forlano, Sisneros, Rohmann, & Bass, 2015 Bose, CogliaQ, Howe, Balshine, 2014 1 5/4/16 What proporEon of Type I males Paternal care occurs in Glass Frogs engaged in cannibalism of eggs? Obligate vs. facultaQve care Grey bars: cannibalisQc males; white bars: noncannibalisQc males Hyalinobatrachium fleischmanni, Family Centrolenidae Delia, Ramírez-‐BauQsta, & Summers, 2014 Bose, CogliaQ, Howe, Balshine, 2014 hOp://www.sciencemag.org/news/2014/04/when-‐dads-‐go-‐missing-‐frogs-‐start-‐hatching Biparental care, monogamy & matrophy! All/most? Female frogs in the family Ranitomeya imitator, Family DendrobaQdae, the poison dart frogs HemiphracEdae a6ach eggs to their backs Stefania ayangannae Brown, Morales, Summers, 2010 2 5/4/16 Tadpoles or direct development with a6ached eggs A frog that gives birth to tadpoles! Limnonectes larvaepartus, Family Dicroglossidae (Fanged Frogs) Pipa carvalhoi PipaFernandes pipa, Antoniazzi, Sasso-‐Cerri, et al. -

Check List 8(2): 207-210, 2012 © 2012 Check List and Authors Chec List ISSN 1809-127X (Available at Journal of Species Lists and Distribution
Check List 8(2): 207-210, 2012 © 2012 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution Amphibians and Reptiles from Paramakatoi and Kato, PECIES S Guyana OF ISTS 1* 2 L Ross D. MacCulloch and Robert P. Reynolds 1 Royal Ontario Museum, Department of Natural History. 100 Queen’s Park, Toronto, Ontario M5S 2C6, Canada. 2 National Museum of Natural History, USGS Patuxent Wildlife Research Center, MRC 111, P.O. Box 37012, Washington, D.C. 20013-7012, USA. * Corresponding author. E-mail: [email protected] Abstract: We report the herpetofauna of two neighboring upland locations in west-central Guyana. Twenty amphibian and 24 reptile species were collected. Only 40% of amphibians and 12.5% of reptiles were collected in both locations. This is one of the few collections made at upland (750–800 m) locations in the Guiana Shield. Introduction palm stands (Maurita flexuosa) (Hollowell et al. 2003). The Guiana Shield region of northeastern South America Immediately west of Kato is the nearby Chiung River, is one of the world’s areas of greatest biodiversity. The a rocky-bottomed watercourse about 50 m wide with herpetofauna of the region remains poorly documented, numerous small falls and a distinct riparian zone. Many although there have been several general publications small agricultural clearings, in typical rotating “slash and on the subject (Starace 1998; Gorzula and Señaris 1999; burn” fashion, are common around Kato in the areas where Lescure and Marty 2000; Reynolds et al. 2001; Avila- savanna transitions to forest. -

Anura | Eleutherodactylidae | Adelophryne Hoogmoed & Lescure, 1984 Fig. 101. Adelophryne Gutturosa Hoogmoed & Lescure, 1
Anura | Eleutherodactylidae | Adelophryne Hoogmoed & Lescure, 1984 Fig. 101. Adelophryne gutturosa Hoogmoed & Lescure, 1984. A. Dorsolateral view of male. B. Ventral surface of a male in life. C. Palm (preserved male specimen). D. Sole (preserved male specimen). E. Call, oscillogram. F. Call, spectrogram. (Photos by P. J. R. Kok). 151 11880-08_ABC-taxa5_01.indd880-08_ABC-taxa5_01.indd 115151 222-01-20092-01-2009 111:13:091:13:09 Anura | Hemiphractidae | Stefania Rivero, 1968 Stefania Rivero, 1968 “STEFANIAS” Fig. 102. Stefania roraimae, a species that does not occur in Kaieteur National Park; here from Mt Maringma. (Photo by P. J. R. Kok). Medium to large size Maxillary teeth present Pupil horizontally elliptical (Fig. 42A) Skin on dorsum smooth, shagreened, granular or tuberculate (Fig. 44A-D) Vocal sac absent (no vocal slits, Fig. 53) Fingers unwebbed Finger discs expanded (Fig. 51B) Finger I > II when fingers adpressed Toe V > III when toes adpressed Tympanum present, distinct (Fig. 43A) Frontoparietal and supratympanic crests absent or present (Fig. 41) 152 11880-08_ABC-taxa5_01.indd880-08_ABC-taxa5_01.indd 115252 222-01-20092-01-2009 111:13:121:13:12 Anura | Hemiphractidae | Stefania Rivero, 1968 The genus currently contains 18 species assigned to two different species groups: the Stefania evansi group (“narrow-headed”) and the S. goini group (“broad-headed”). Stefanias are nocturnal, terrestrial or arboreal. They inhabit tropical rainforest, high-tepui forest and tepui bog. Sexual dimorphism Males are distinctly smaller than females; there is no other evident sexual dimorphism or dichromatism. Eggs Eggs and neonates are carried on the back of the female, adhering to a mucus layer. -

Download Download
Phyllomedusa 7(2):143-148, 2008 © 2008 Departamento de Ciências Biológicas - ESALQ - USP ISSN 1519-1397 Ovipositing behavior in the egg-brooding frog Stefania ayangannae (Anura, Hemiphractidae) D. Bruce Means1, William E. Duellman2 and Valerie C. Clark3 1 Coastal Plains Institute and Land Conservancy, 1313 Milton Street, Tallahassee, FL 32303, USA. E-mail: [email protected]. 2 Natural History Museum and Biodiversity Research Center, University of Kansas, 1345 Jayhawk Blvd., Lawrence, Kansas 66045, USA. E-mail: [email protected]. 3 School of Pharmacy, Queen’s University in Belfast, Belfast BT9 7BL, Northern Ireland, UK. E-mail: [email protected]. Keywords: Anura, Hemiphractidae, Stefania ayangannae, ovipositing behavior, Wokomung Massif, Guyana. Palabras clave: Anura, Hemiphractidae, Stefania ayangannae, comportamiento de la postura, Wokomung Massif, Guyana. Frogs of the family Hemiphractidae, as for at least 6 of the 18 described species (Rivero recognized by Wiens et al. (2007), are unique 1968, Duellman and Hoogmoed 1984, Señaris among anurans in that the eggs develop on the et al. 1997, MacCulloch and Lathrop back or in dorsal pouches in the female 2006a,b,c). Oviposition, male behavior during (Duellman and Maness 1980). Females of fertilization, and placement of eggs on the species of Cryptobatrachus, Hemiphractus, and female’s dorsum have been described in one Stefania carry their eggs and young on their captive pair of Stefania (species not identified backs; the eggs are attached to the body by a but probably S. riveroi) from Yuruani tepui in glutinous material (Jungfer and Boehme 1991). Venezeula (Magdefrau 1991). Herein we In other hemiphractids, the eggs develop in a describe these behaviors in a wild pair of S. -

Museum of Comparative Zoology
BREVIOR A Museum of Comparative Zoology US ISSN 0006-9698 Cambridge, Mass. 18 April 1996 Number 506 A PHENACOSAUR FROM CHIMANTA TEPUI, VENEZUELA Ernest E. Williams, 1 Maria Jose Praderio, 2 and Stefan Gorzula 3 Abstract. A new species of the genus Phenacosaurus is described from Chi- manta Tepui, close to P. neblininus. It differs from P. neblininus (and other known phenacosaurs) in having the interparietal smaller than the ear and in having the circumnasal in broad contact with the rostral and only barely touching or not all in contact with the first supralabial. It also differs from neblininus in a generally darker coloration and having the belly with bold dark reticulation. INTRODUCTION Until Lazell (1969) described the new species Phenacosaurus orcesi from two localities in Ecuador, the anoline lizards separated as the genus Phenacosaurus had been known only from Colombia and from just over the border in Venezuela. A summary of new information has been reported in Williams et al. (1996). Now still another small but distinctive new species, represented by a unique specimen, deposited in the collections of the Sociedad de Ciencias Naturales “La Salle,” Caracas, most similar to the other tepui species, P. neblininus, from Cerro de La Neblina, provides the easternmost representative of the genus from Chi- manta Tepui in Venezuela. The new species is named after the late Carlos Todd, long active in conservation work (Gorzula, 1987), who participated in the exploration of Chimanta Tepui that resulted in the discovery of the new species Phenacosaurus carlostoddi. 1 Museum of Comparative Zoology, Harvard University, Cambridge, Massachu- setts 02138. -

Reevolution of Lost Mandibular Teeth in Frogs
ORIGINAL ARTICLE doi:10.1111/j.1558-5646.2011.01221.x RE-EVOLUTION OF LOST MANDIBULAR TEETH IN FROGS AFTER MORE THAN 200 MILLION YEARS, AND RE-EVALUATING DOLLO’S LAW John J. Wiens Department of Ecology and Evolution, Stony Brook University, Stony Brook, NY 11794-5245 E-mail: [email protected] Received June 24, 2010 Accepted December 7, 2010 Dollo’s law states that structures that are evolutionarily lost will not be regained. Recent phylogenetic studies have revealed several potential examples in which Dollo’s law seems to be violated, where lost structures appear to have been regained over evolutionary time. However, these examples have recently been questioned and suggested to be methodological artifacts. In this article, I document a striking and incontrovertible phylogenetic example of the re-evolution of a lost, complex structure: mandibular teeth in the frog genus Gastrotheca. I use a time-calibrated phylogeny for 170 amphibian species to show that mandibular teeth were lost in the ancestor of modern frogs at least 230 million years ago (Mya) and have been regained in the last ∼5–17 My. I review recent studies on trait re-evolution and show that this long period of trait absence prior to re-acquisition is largely unprecedented. I also argue that there are several methodological issues that may cause trait re-evolution to be hardest to detect under those conditions when it is most likely to occur, leading to erroneous failures to reject Dollo’s law. Finally, I discuss a mechanism that may facilitate trait re-evolution, and the evolution of mandibular teeth in frogs as an example of developmental constraint. -

Revista 7-2 Jul-Dez 2008 15 12 2008.P65
Phyllomedusa 7(2):143-148, 2008 © 2008 Departamento de Ciências Biológicas - ESALQ - USP ISSN 1519-1397 Ovipositing behavior in the egg-brooding frog Stefania ayangannae (Anura, Hemiphractidae) D. Bruce Means1, William E. Duellman2 and Valerie C. Clark3 1 Coastal Plains Institute and Land Conservancy, 1313 Milton Street, Tallahassee, FL 32303, USA. E-mail: [email protected]. 2 Natural History Museum and Biodiversity Research Center, University of Kansas, 1345 Jayhawk Blvd., Lawrence, Kansas 66045, USA. E-mail: [email protected]. 3 School of Pharmacy, Queen’s University in Belfast, Belfast BT9 7BL, Northern Ireland, UK. E-mail: [email protected]. Keywords: Anura, Hemiphractidae, Stefania ayangannae, ovipositing behavior, Wokomung Massif, Guyana. Palabras clave: Anura, Hemiphractidae, Stefania ayangannae, comportamiento de la postura, Wokomung Massif, Guyana. Frogs of the family Hemiphractidae, as for at least 6 of the 18 described species (Rivero recognized by Wiens et al. (2007), are unique 1968, Duellman and Hoogmoed 1984, Señaris among anurans in that the eggs develop on the et al. 1997, MacCulloch and Lathrop back or in dorsal pouches in the female 2006a,b,c). Oviposition, male behavior during (Duellman and Maness 1980). Females of fertilization, and placement of eggs on the species of Cryptobatrachus, Hemiphractus, and female’s dorsum have been described in one Stefania carry their eggs and young on their captive pair of Stefania (species not identified backs; the eggs are attached to the body by a but probably S. riveroi) from Yuruani tepui in glutinous material (Jungfer and Boehme 1991). Venezeula (Magdefrau 1991). Herein we In other hemiphractids, the eggs develop in a describe these behaviors in a wild pair of S. -

IACUC Update
IACUC Update IACUCInstitutional Animal Care and Use Committee Update The University of Texas at Austin July 2014 Volume 7, Issue 10 “Introduction to Wildlife” offered Meeting Requirements for in the AALAS Learning Library Alternative Searches: Webinar Do you conduct studies with wildlife in Available for Viewing research? If so, there is a new AALAS A recording of the OLAW Online Seminar Learning Library (ALL) module “Introduction “Meeting Requirements for Alternatives to Wildlife.” This module will provide Searches: Information for IACUCs and information on: Investigators” broadcast on June 26, 2014, Protocol review issues has been posted on the OLAW website.- Lorem Ipsum • • Occupational health issues In this webinar, Kathleen Gregory, reference • Transportation and holding of wild librarian at the University of Denver, caught animals provided information for IACUCs and • Capture methods researchers about the alternatives search • Marking or identification methods required by the USDA Animal Welfare • Proper permits from local and federal Regulations. agencies The recording and supporting materials can • Recognition and management of pain be found on the Education Resources and distress in wild animals webpage. • Euthanasia, and more! When you view the webinar, please email At the July 14, 2014 Full Committee Review, Justin McNulty ([email protected]) the IACUC voted to require this module for all so that your TXClass training record can be non-fish, non-amphibian, non-traditional documented. laboratory animal users, effective December 1, 2014. For a specific listing of affected species, please refer to the attached listing. eProtocol Tip of the Month On December 1, 2014, all species listed in the Remember: Protocols, Amendments, and attached listing will be coded in eProtocol for Continuing Review applications cannot be this course. -

AMPHIBIA: ANURA: CRYPTOBATRACHIDAE Stefania Woodleyi
AMPHIBIA: ANURA: CRYPTOBATRACHIDAE Stefania woodleyi Catalogue of American Amphibians and Reptiles. to granular. Rounded warts are present in the tempo- ral and post-tympanic regions, with low warts also MacCulloch, R.D. and A. Lathrop. 2006. Stefania present in the loreal region. The largest finger disc is woodleyi. equal to half the tympanum diameter. The hands and feet have low, indistinct supernumerary tubercles. Stefania woodleyi Rivero Toe webbing formula is 1 (2+-2%) - 2%11 (2-2) - (3-3+) Woodley's Stefania; Rana Stefania de 111 (2%-2%) - (3&3%) IV 3%- (2+-2%) v. Woodley Dorsal surfaces are medium brown with irregular ochre spots or reticulations. Some individuals have an ochre or cream interorbital bar and dorsolateral Stefania woodleyi Rivero 1968:147. Type-locality, stripes. Laterally, the head is ochre with a dark brown '...from a rocky stream, slope Mt. Kanaima, nr. canthal stripe and irregular medium brown spots in Potaro R. Brit. Guiana." Holotype, British Museum loreal and temporal regions. A dark brown supratym- of Natural History (BMNH) 1967.654, adult fe- panic stripe continues to the groin. Flanks and groin male, collected 17 August 1959 by J.D. Woodley are medium to dark brown with irregular ochre spots. (examined by authors). Dorsal surfaces of thighs and arms are ochre with dark brown transverse bars continuing onto anterior CONTENT. No subspecies are recognized. surfaces of thighs; posterior surfaces of thighs are medium brown with white spots. Shanks and tarsi are DEFINITION. Adults range in SVL from 35-46 mm ochre with dark brown spots or bars. Throat and ven- (males) and 44-60 mm (females).