Activity and Homing Behavior of Two Species of Acanthopleura (Mollusca: Polyplacophora) on a Subtropical Shore in Japan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Polyplacophora: Chitonidae): First Records in European Waters
Zootaxa 3626 (4): 593–596 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Correspondence ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3626.4.14 http://zoobank.org/urn:lsid:zoobank.org:pub:00EE2336-D60C-49A1-BC40-0FAE551F5DB6 Tonicia atrata and Chiton cumingsii (Polyplacophora: Chitonidae): First records in European waters ANDRÉS ARIAS1,2 & NURIA ANADÓN1 1Departamento de Biología de Organismos y Sistemas (Zoología), Universidad de Oviedo, Oviedo 33071, Spain 2Corresponding author. E-mail: [email protected] At present, over 300 species of marine alien Mollusca are reported from the European waters (Streftaris et al. 2005; Zenetos et al. 2010). However, only three alien polyplacophoran have been recorded: Chaetopleura angulata (Spengler, 1797), Acanthopleura gemmata (Blainville, 1825) and Chiton hululensis (E. A. Smith, 1903); the latter is considered as “questionable” (Zenetos et al. 2010). These polyplacophoran constituting about 1% of the alien marine mollusc reported from Europe. Here we present the first record of Tonicia atrata (Sowerby, 1840) and Chiton cumingsii Frembly, 1827 in European waters, constituting the first evidence of their presence outside their native range. Furthermore, we give brief notes on the taxonomy and distribution of T. atrata and C. cumingsii, and discuss the potential pathways for introduction to Europe. In Europe, T. atrata occurs together with the well-known alien Ch. angulata; and probably both species have historically been misidentified in collections because both reach large size (> 60 mm) and in many cases the larger size was commonly used to differentiate the presumed alien (Ch. angulata) from the native polyplacophoran of smaller size. -

Download Preprint
1 Mobilising molluscan models and genomes in biology 2 Angus Davison1 and Maurine Neiman2 3 1. School of Life Sciences, University Park, University of Nottingham, NG7 2RD, UK 4 2. Department of Biology, University of Iowa, Iowa City, IA, USA and Department of Gender, 5 Women's, and Sexuality Studies, University of Iowa, Iowa, City, IA, USA 6 Abstract 7 Molluscs are amongst the most ancient, diverse, and important of all animal taxa. Even so, 8 no individual mollusc species has emerged as a broadly applied model system in biology. 9 We here make the case that both perceptual and methodological barriers have played a role 10 in the relative neglect of molluscs as research organisms. We then summarize the current 11 application and potential of molluscs and their genomes to address important questions in 12 animal biology, and the state of the field when it comes to the availability of resources such 13 as genome assemblies, cell lines, and other key elements necessary to mobilising the 14 development of molluscan model systems. We conclude by contending that a cohesive 15 research community that works together to elevate multiple molluscan systems to ‘model’ 16 status will create new opportunities in addressing basic and applied biological problems, 17 including general features of animal evolution. 18 Introduction 19 Molluscs are globally important as sources of food, calcium and pearls, and as vectors of 20 human disease. From an evolutionary perspective, molluscs are notable for their remarkable 21 diversity: originating over 500 million years ago, there are over 70,000 extant mollusc 22 species [1], with molluscs present in virtually every ecosystem. -

<I>Acanthopleura Gemmata</I>
NOTES 339 Mauri, M. and E. Orlando. 1983. Variability of zinc and manganese concentrations in relation to sex and season in the bivalve Donax trunculus. Mar. Pollut. Bull. 14: 342-346. McConchie, D. and L. M. Lawrance. 1991. The origin of high cadmium loads in some bivalves molluscs from Shark Bay, Western Australia: a new mechanism for cadmium uptake by filter feeding organisms. Arch. Environ. Contam. Toxicol. 21: 303-310. Nugegoda, D. and P. S. Rainbow. 1987. The effect of temperature on zinc regulation by the decapod crustacean Palaemon elegans Rathke. Ophelia 27: 17-30. Orlando, E. ]985. Valutazione dell'inquinamento marino da metalli pesanti tramite ]'uso di indicatori biologici. Oebalia XI: 93-100. Orren, M. J., G. A. Eagle, H. F.-K. O. Hennig and A. Green. 1980. Variations in trace metal content of the mussel Choromytilus meridionalis (Kr.) with season and sex. Mar. Pollut. Bull. 11: 253- 257. Rainbow, P. S. and A. G. Scott. 1979. Two heavy metal-binding proteins in the midgut gland of the crab Carcinus maenas. Mar. BioI. 55: 143-150. DATE ACCEPTED: April 13, ]994. ADDRESSES: (K.F.) Western Australian Marine Research Laboratories, PO Box 20, North Beach 6020, Australia; (CS.) Chemistry Centre of Western Australia, 125 Hay Street, East Perth 6004, Australia; (M.J.) Health Department of Western Australia, 189 Royal Street, East Perth 6004, Aus- tralia. BULLETINOF MARINESCIENCE,56( I): 339-343, 1995 KARYOLOGICAL STUDIES ON THE COMMON ROCKY EGYPTIAN CHITON, ACANTHOPLEURA GEMMATA (POLYPLACOPHORA: MOLLUSCA) Ahmed E. Yaseen, Abdel-Baset M. Ebaid and I. S. Kawashti Acanthopleura gemmata (Blainville, 1925) is one of the commonest polypla- cophoran species and is very common along the Egyptian coasts (northwestern part of the Red Sea) (Soliman and Habib, 1990). -

The Coastal Molluscan Fauna of the Northern Kermadec Islands, Southwest Pacific Ocean
Journal of the Royal Society of New Zealand ISSN: 0303-6758 (Print) 1175-8899 (Online) Journal homepage: http://www.tandfonline.com/loi/tnzr20 The coastal molluscan fauna of the northern Kermadec Islands, Southwest Pacific Ocean F. J. Brook To cite this article: F. J. Brook (1998) The coastal molluscan fauna of the northern Kermadec Islands, Southwest Pacific Ocean, Journal of the Royal Society of New Zealand, 28:2, 185-233, DOI: 10.1080/03014223.1998.9517560 To link to this article: http://dx.doi.org/10.1080/03014223.1998.9517560 Published online: 30 Mar 2010. Submit your article to this journal Article views: 405 View related articles Citing articles: 14 View citing articles Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=tnzr20 Download by: [86.95.75.143] Date: 27 January 2017, At: 05:34 © Journal of The Royal Society of New Zealand, Volume 28, Number 2, June 1998, pp 185-233 The coastal molluscan fauna of the northern Kermadec Islands, Southwest Pacific Ocean F. J. Brook* A total of 358 species of molluscs (excluding pelagic species) is recorded here from coastal marine habitats around the northern Kermadec Islands. The fauna is dominated by species that are widely distributed in the tropical western and central Pacific Ocean. The majority of these are restricted to the tropics and subtropics, but some range south to temperate latitudes. Sixty-eight species, comprising 19% of the fauna, are thought to be endemic to the Kermadec Islands. That group includes several species that have an in situ fossil record extending back to the Pleistocene. -

(Polyplacophora, Chitonidae) in the Southwestern Atlantic Ocean
Rev. Mus. Argentino Cienc. Nat., n.s. 21(1): 1-6, 2019 ISSN 1514-5158 (impresa) ISSN 1853-0400 (en línea) First records of coalescence and hypomerism in Tonicia atrata (Polyplacophora, Chitonidae) in the southwestern Atlantic Ocean Camila GUILLÉN1 & Diego URTEAGA2 1Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata. Avenida 122 y 60, (1900) La Plata, Argentina. 2Museo Argentino de Ciencias Naturales, CONICET. Av. Ángel Gallardo 470, (C1405DJR) Ciudad Autónoma de Buenos Aires, Argentina; e-mail: [email protected]. Abstract: The first cases of anomalies in the number of shell-plates in Tonicia atrata (Sowerby II, 1840) are herein reported on the basis of three specimens from Zaratiegui Bay, Tierra del Fuego, Argentina (54°50’53’’ S, 68°29’7’’ W): two cases of coalescence (one symmetrical and another one asymmetrical) and a case of hypomerism. The abnormal specimens bore less ctenidia than physiological conspecific specimens of similar size. The symme- trical coalescent specimen and the hypomeric individual were significantly wider (higher width/length ratio) than physiological specimens. Nevertheless, the reported abnormalities seem to have no effect on general growth, since the body size of the abnormal specimens were close to the largest physiological ones found in the same locality. Key words: teratology, morphology, Mollusca, Argentina, Patagonia. Resumen: Primeros registros de coalescencia e hipomerismo en Tonicia atrata (Polyplacophora, Chitonidae) en el Océano Atlántico sudoccidental. Se registran aquí los primeros casos de anomalías en el número de placas en ejemplares de Tonicia atrata (Sowerby II, 1840), sobre la base de tres ejemplares hallados en Bahía Zaratiegui, Tierra del Fuego, Argentina (54°50’53’’ S, 68°29’7’’ O): dos casos de coalescencia (uno simétrico; el otro asimétrico) y un caso de hipomerismo. -

Molluscan Studies
Journal of The Malacological Society of London Molluscan Studies Journal of Molluscan Studies (2013) 79: 372–377. doi:10.1093/mollus/eyt029 Advance Access publication date: 23 August 2013 RESEARCH NOTE EGG-HULL ULTRASTRUCTURE OF ISCHNOCHITON STRAMINEUS (SOWERBY, 1832), A SOUTH AMERICAN BROODING CHITON (CHITONINA: ISCHNOCHITONIDAE) Marı´a G. Liuzzi1 and Diego G. Zelaya2 1Divisio´n Invertebrados, Museo Argentino de Ciencias Naturales, CONICET, A´ngel Gallardo 470, Buenos Aires (C1405DJR), Argentina; and 2Departamento Biodiversidad, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, CONICET, Argentina Correspondence: M.G. Liuzzi; e-mail: [email protected] Reproductive aspects of the life history of chitons have been rela- Celebron˜a(¼Kidney) Island, Malvinas/Falkland Islands tively little studied in comparison with some morphological (518370S–578450W). Thirty-eight eggs were removed from the characters, such as valve morphology, girdle scales and radulae, pallial groove, dehydrated in an ascending ethanol series (from which have historically been considered of taxonomic value. In 70 to 100%), dried in an EMS 850 critical-point dryer using recent times, however, some authors have pointed out that char- liquid CO2, coated with gold-palladium (40–60%) and photo- acters related to reproduction, such as egg-hull morphology, are graphed with a Phillips XL-30 scanning electron microscope. also valuable for taxonomic purposes (Eernisse, 1988; Hodgson One juvenile was dehydrated and photographed in the same et al., 1988; Sirenko, 1993; Pashchenko & Drozdov, 1998; Okusu way as the eggs, but treated with hexamethyldisilazane and air et al., 2003; Buckland-Nicks, 2006, 2008; Sirenko, 2006a; dried instead of critical-point dried. -

Polyplacophora: the Sesoko Gtation
The malacological society of Japan VEN US Jour. MHIac.} Hn (Jap, - Vol. 5S, Nu. 1 c1996}/ 41 49 -vs mazanog= u 7 ti l K 4 twoi)ts - itimaptL ptideptkA ・ ( pan-E11i!;( ftcma j(4}!: J,!UKJI(A7mpt}ttpt) of Distribution of Four Species Acanthopteura in Sesoko Island, Okinawa' (Polyplacophora:Chitonidae) Eiji YosHIoKA and Yasuhiro NAKAsHIMA (Kobe Yamate Women's College, Nakayamate-dori 6, Kobe 650 and Faculty of Science, Kyoto University, Kyoto 606-Ol) Abstract: Horizontal and vertical distribution of chitons, Acanthopleura gemmata. A. have been investigated. In the horizontal aspect, toochooana.A. tenuispinosa and A. miles, in the sheltered-exposed A. distributes they have specific distribution gradation. gemmata all rock shores around the island. A. Ioochooana distributes almost al1 around the island area. A. tenuispinosa mainly distributes the most sheltered area. except the most exposed In the vertical aspect, their A, miies mainly distributes the most exposed area. habitats significant except between are mostly overlapping, but their mean height have difference is lower, that of A. A. tenuispinosa and A. mites. The mean height of A, gemmata toochooana is middle, those of A, tenuispinosa and A, miles are higher in their habitat counts together, those species have specific zone. Puttingthe horizontal and vertical a ¢ four `space sharing' the dimensions although their habitat area and range overlap in in twe the most cases. Introduction Ferreira (1986) reviewed the genus Acanthopleura elaborately, and surnmarized the distri- bution of Acanthqpleura spp. According to his article, the distribution of three species, A. gemrnata (Fig. 1-A), A. Ioochooana (Fig. 1-B) and A. -
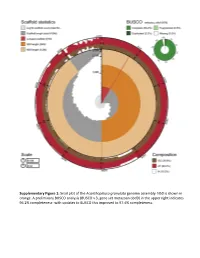
Snail Plot of the Acanthopleura Granulata Genome Assembly
Supplementary Figure 1: Snail plot of the Acanthopleura granulata genome assembly. N50 is shown in orange. A preliminary BUSCO analysis (BUSCO v.3, gene set metazoan obd9) in the upper right indicates 96.2% completeness- with updates to BUSCO this improved to 97.4% completeness. 1e+03 ● 1e+01 Mb N50), log(scaffold log(scaffold 1e−01 1e−03 20 40 60 80 100 BUSCO score, % Supplementary Figure 2: An illustration of the quality of available molluscan genomes as a scatter plot of BUSCO completeness score and a log transform of the N50 of scaffolds in megabases for each genome. The A. granulata genome is the orange circle, bivalves are purple triangles, gastropods are green squares, and cephalopods are blue stars. Grey diamonds are representative genomes of other lophotrochozoans (specifically those used as outgroups in the homolog gene searches in this study). 100 Acanthopleura granulata 93 Acanthopleura gemmata Rhyssoplax olivaceus 100 Chitonina 100 Chiton marmoratus Chitonida 100 Lepidozona mertensii 100 Katharina tunicata Mopalia muscosa Polyplacophora 100 100 Nuttallochiton mirandus Acanthochitonina 100 Cryptoplax larvaeformis 100 100 Acanthochitona crinita Callochiton sp. Leptochiton asellus Lepidopleurida 100 Hanleya nagelfar Prochaetoderma californicum 100 100 Chaetoderma nitidulum Caudofoveata 100 Falcidens sagittiferus Scutopus ventrolineatus 84 Epimenia babai 100 54 100 Apodomenia enigmatica 100 Neomenia megatrapezata Solenogastres 100 Stylomenia sulcodoryata 100 Amphimeniidae sp. 100 Octopus bimaculoides 100 Euprymna scolopes Cephalopoda -

Curaçao and Other
STUDIES ON THE FAUNA OF CURAÇAO AND OTHER CARIBBEAN ISLANDS: No. 137. Polyplacophora of the Caribbean Region by P. Kaas (Zoologisch Laboratorium, Utrecht) Contents Page Figure Plate INTRODUCTION 3 Materials 4 Historical review 5 Distribution (Tables 1 & 2) 8 SYSTEM ATICS 14 Lepidopleurida 15 Lepidopleuridae 15 Lepidopleurus Risso, 1826 16 1 pergranatus (Dall, 1889) 16 1-6 2 — binghami Boone, 1928 18 7-12 Hanleya Gray, 1857 20 3 — tropicalis (Dall, 1881) 20 13-18 Chitonida 21 Lepidochitonidae 22 Lepidochitona Gray, 1821 22 4 22 1 liozonis (Dall & Simpson, 1901) .... 19-40 I, 5 - rosea sp. n 27 41-49 Mopaliidae 29 Ceratozona Dall, 1882 29 6 rugosa (Sowerby, 1840) 29 50-54 1,2-3 Cryptoplacidae 33 Cryptoconchus Burrow, 1815 34 7 - floridanus (Dall, 1889) 34 55-57 I, 4-5 2 Page Figure Plate Acanthochitona Gray, 1821 37 8 hemphilli {Pilsbry, 1893) 38 58-64 II, 1-2 9 rhodea (Pilsbry, 1893) 42 65-71 bonairensis 44 72-73 10 n.sp III, 1-2 11 spiculosa (Reeve, 1847) 46 74-81 12 pygmaea (Pilsbry, 1893) 49 82-89 13 - 51 90-94 elongata n.sp 11,3 - 53 14 interfissa n.sp 95-107 Choneplax (Carpenter MS) Dall, 1882 . 55 15 lata (Guilding, 1829) 55 108-116 11,4 Ischnochitonidae 58 Calloplax Thiele, 1909 59 16 janeirensis (Gray, 1828) 60 117-123 IV, 1-2 Chaetopleura Shuttleworth, 1853 .... 63 17 apiculata (Say, 1830) 63 124-128 IV, 3-6 Ischnochiton Gray, 1847 66 B. 68 129-134 18 purpurascens (C. Adams, 1845) ... V, 1 19 - floridanus Pilsbry, 1892 72 135-136 V, 2 20 — boogii Haddon, 1886 74 137—150 21 - striolatus Gray, 1828 77 151-166 V, 3-4 - B. -

Mollusca: Polyplacophora)
Research Ideas and Outcomes 6: e60446 doi: 10.3897/rio.6.e60446 Project Report Large-scale project ‘Chiton of the Mexican Tropical Pacific’: Chiton articulatus (Mollusca: Polyplacophora) Omar Hernando Avila-Poveda ‡, § ‡ Facultad de Ciencias del Mar (FACIMAR), Universidad Autonoma de Sinaloa (UAS), Mazatlan, Sinaloa, Mexico § Direccion de Catedras-CONACYT, Consejo Nacional de Ciencia y Tecnologia (CONACYT), Ciudad de Mexico, Mexico Corresponding author: Omar Hernando Avila-Poveda ([email protected]) Reviewable v 1 Received: 06 Nov 2020 | Published: 10 Nov 2020 Citation: Avila-Poveda OH (2020) Large-scale project ‘Chiton of the Mexican Tropical Pacific’: Chiton articulatus (Mollusca: Polyplacophora). Research Ideas and Outcomes 6: e60446. https://doi.org/10.3897/rio.6.e60446 Abstract The marine mollusc, commonly called sea cockroach or chiton Chiton articulatus, is a mollusc belonging to the group known as Polyplacophora because its shell is composed of eight individual plates. This mollusc inhabits the rocky intertidal shore of the Mexican Tropical Pacific, where it is endemic. It has ecological, but also economic, importance. Ecologically, it is the preferred food of the snail Plicopurpura pansa, a protected species, in the cultural heritage of the country. Additionally, it is a basibiont (generates substrate for other individuals) that maintains the biodiversity of the Region. Economically, it has changed from artisanal consumption to become a culinary tourist attraction, offered at restaurants as an exotic and aphrodisiac dish, in tourist places like Huatulco or Acapulco. Despite being an exploited resource for decades, little is known about its life history. The Mexican Authorities have not yet recognised this mollusc as a fishing resource, so that it does not have any law that controls its extraction, sale and consumption, putting at risk the recruitment, survival and permanence of this species. -

Illustrated Summary of Chiton Terminology
©Zoologische Staatssammlung München/Verlag Friedrich Pfeil; download www.pfeil-verlag.de SPIXIANA 33 2 171–194 München, November 2010 ISSN 0341–8391 Illustrated summary of chiton terminology (Mollusca, Polyplacophora) Enrico Schwabe Schwabe, E. 2010. Illustrated summary of chiton terminology (Mollusca, Poly- placophora). Spixiana 33 (2): 171-194. The aims of the present paper are to summarize and offer a standard set of ter- minology used to describe morphological and partly anatomical (e. g. the radula) characters of Polyplacophora. To make the understanding of some previously misused terms easier, the identification and description of relevant parts of the animal are illustrated and discussed in context of additional literature. Enrico Schwabe, Bavarian State Collection of Zoology, Muenchhausenstr. 21, 81247 Munich, Germany; e-mail: [email protected] Introduction “An den Tentakeln des Kopfes befinden sich Riech- organe [On the tentacles of the head there are Chitons are a group of basal, exclusively marine olfactory organs.].” However, chitons do not have molluscs, which have not significantly changed cephalic tentacles at all, and certainly not olfactory their bauplan during more than 300 million years tentacles! of evolution. Their more or less solid, dorsal plates This short contribution intends to clarify the termi- have been preserved in numerous fossil records and nology of chitons and to present a detailed general allow researchers a direct comparison with living description of chiton morphology, to summarise for species, and accordingly there is a high number amateurs, students or scientists the present stage of described fossil and recent taxa. At present we of scientific knowledge of a fascinating group of count for about 930 recent species (see Schwabe animals. -

Foraging Tactics in Mollusca: a Review of the Feeding Behavior of Their Most Obscure Classes (Aplacophora, Polyplacophora, Monoplacophora, Scaphopoda and Cephalopoda)
Oecologia Australis 17(3): 358-373, Setembro 2013 http://dx.doi.org/10.4257/oeco.2013.1703.04 FORAGING TACTICS IN MOLLUSCA: A REVIEW OF THE FEEDING BEHAVIOR OF THEIR MOST OBSCURE CLASSES (APLACOPHORA, POLYPLACOPHORA, MONOPLACOPHORA, SCAPHOPODA AND CEPHALOPODA) Vanessa Fontoura-da-Silva¹, ², *, Renato Junqueira de Souza Dantas¹ and Carlos Henrique Soares Caetano¹ ¹Universidade Federal do Estado do Rio de Janeiro, Instituto de Biociências, Departamento de Zoologia, Laboratório de Zoologia de Invertebrados Marinhos, Av. Pasteur, 458, 309, Urca, Rio de Janeiro, RJ, Brasil, 22290-240. ²Programa de Pós Graduação em Ciência Biológicas (Biodiversidade Neotropical), Universidade Federal do Estado do Rio de Janeiro E-mails: [email protected], [email protected], [email protected] ABSTRACT Mollusca is regarded as the second most diverse phylum of invertebrate animals. It presents a wide range of geographic distribution patterns, feeding habits and life standards. Despite the impressive fossil record, its evolutionary history is still uncertain. Ancestors adopted a simple way of acquiring food, being called deposit-feeders. Amongst its current representatives, Gastropoda and Bivalvia are two most diversely distributed and scientifically well-known classes. The other classes are restricted to the marine environment and show other limitations that hamper possible researches and make them less frequent. The upcoming article aims at examining the feeding habits of the most obscure classes of Mollusca (Aplacophora, Polyplacophora, Monoplacophora, Scaphoda and Cephalopoda), based on an extense literary research in books, journals of malacology and digital data bases. The review will also discuss the gaps concerning the study of these classes and the perspectives for future analysis.