Maternal Lines Using Mitochondrial D-Loop Sequence Variation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

USDA-NRSP8 Report for Horse Genome Committee for January 1, 2014 to December 31, 2014
USDA-NRSP8 Report for Horse Genome Committee for January 1, 2014 to December 31, 2014 Coordinators: Ernest Bailey (University of Kentucky); Molly McCue (University of Minnesota), Samantha Brooks (University of Florida) Workshop Chair for 2015 PAG meeting: Scott Dindot (Texas A&M University) Workshop Chair for 2016 PAG meeting: Ted Kalbfleisch (University of Louisville) Workshop Chair for 2017 PAG meeting: Carrie Finno (University of California, Davis) The workshop participants met on Saturday and Sunday, January 10-11, 2015 at the Plant and Animal Genome Conference in San Diego. Approximately 80 people attended the sessions with participants from at least 10 countries (USA, Brazil, China, Japan, Korea, Denmark, United Kingdom, Italy, Argentina, Ireland). Scott Dindot served as chair of the 2015 workshop. He will step down after this year and the next chair will be Ted Kalbfleisch. At the meeting, Carrie Finno was elected as vice-chair and will assist Ted Kalbfleisch in 2016 and assume full leadership of the workshop in 2017. Carrie J. Finno [email protected] 1 530 752 2739 Department of Population Health and Reproduction 280 CCAH University of California, Davis, CA 95616 Objective 1: Advance the status of reference genomes for all species, including basic annotation of worldwide genetic variation, by broad sequencing among different lines and breeds of animals. New Reference Genome Assembly Ted Kalbfleisch announced that the Morris Animal Foundation had selected for funding a proposal crafted by Ted, Jamie MacLeod and Ludovic Orlando for creating a new assembly of the reference sequence, the putative Ecab 3.0. Partial support for a postdoctoral student will come from USDA-NRSP8 coordinators’ funds. -

Population Genetic Analysis of the Estonian Native Horse Suggests Diverse and Distinct Genetics, Ancient Origin and Contribution from Unique Patrilines
G C A T T A C G G C A T genes Article Population Genetic Analysis of the Estonian Native Horse Suggests Diverse and Distinct Genetics, Ancient Origin and Contribution from Unique Patrilines Caitlin Castaneda 1 , Rytis Juras 1, Anas Khanshour 2, Ingrid Randlaht 3, Barbara Wallner 4, Doris Rigler 4, Gabriella Lindgren 5,6 , Terje Raudsepp 1,* and E. Gus Cothran 1,* 1 College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, College Station, TX 77843, USA 2 Sarah M. and Charles E. Seay Center for Musculoskeletal Research, Texas Scottish Rite Hospital for Children, Dallas, TX 75219, USA 3 Estonian Native Horse Conservation Society, 93814 Kuressaare, Saaremaa, Estonia 4 Institute of Animal Breeding and Genetics, University of Veterinary Medicine Vienna, 1210 Vienna, Austria 5 Department of Animal Breeding and Genetics, Swedish University of Agricultural Sciences, 75007 Uppsala, Sweden 6 Livestock Genetics, Department of Biosystems, KU Leuven, B-3001 Leuven, Belgium * Correspondence: [email protected] (T.R.); [email protected] (E.G.C.) Received: 9 August 2019; Accepted: 13 August 2019; Published: 20 August 2019 Abstract: The Estonian Native Horse (ENH) is a medium-size pony found mainly in the western islands of Estonia and is well-adapted to the harsh northern climate and poor pastures. The ancestry of the ENH is debated, including alleged claims about direct descendance from the extinct Tarpan. Here we conducted a detailed analysis of the genetic makeup and relationships of the ENH based on the genotypes of 15 autosomal short tandem repeats (STRs), 18 Y chromosomal single nucleotide polymorphisms (SNPs), mitochondrial D-loop sequence and lateral gait allele in DMRT3. -

List of Horse Breeds 1 List of Horse Breeds
List of horse breeds 1 List of horse breeds This page is a list of horse and pony breeds, and also includes terms used to describe types of horse that are not breeds but are commonly mistaken for breeds. While there is no scientifically accepted definition of the term "breed,"[1] a breed is defined generally as having distinct true-breeding characteristics over a number of generations; its members may be called "purebred". In most cases, bloodlines of horse breeds are recorded with a breed registry. However, in horses, the concept is somewhat flexible, as open stud books are created for developing horse breeds that are not yet fully true-breeding. Registries also are considered the authority as to whether a given breed is listed as Light or saddle horse breeds a "horse" or a "pony". There are also a number of "color breed", sport horse, and gaited horse registries for horses with various phenotypes or other traits, which admit any animal fitting a given set of physical characteristics, even if there is little or no evidence of the trait being a true-breeding characteristic. Other recording entities or specialty organizations may recognize horses from multiple breeds, thus, for the purposes of this article, such animals are classified as a "type" rather than a "breed". The breeds and types listed here are those that already have a Wikipedia article. For a more extensive list, see the List of all horse breeds in DAD-IS. Heavy or draft horse breeds For additional information, see horse breed, horse breeding and the individual articles listed below. -

Hoof Quality of Anglo-Arabian and Haflinger Horses
J Vet Res 61, 367-373, 2017 DE DE GRUYTER OPEN DOI:10.1515/jvetres-2017-0049 G Hoof quality of Anglo-Arabian and Haflinger horses Roberto Tocci, Clara Sargentini, Andrea Martini, Luisa Andrenelli, Antonio Pezzati, Doria Benvenuti, Alessandro Giorgetti Department of Agrifood Production and Environmental Sciences – Animal Science Section University of Florence, 50144 Florence, Italy [email protected] Received: April 20, 2017 Accepted: August 18, 2017 Abstract Introduction: Foot quality is essential to the horse’s movement. The barefoot approach favours the animal’s welfare. Environment and selection determine hoof characteristics. Material and Methods: Hoof characteristics of eight Anglo-Arabian (AA) and nine Haflinger (HA) horses were studied. After a preliminary visual analysis of feet, nail samples were collected after trimming for physico-chemical analysis. The parameters were submitted to analysis of variance. A principal component analysis and a Pearson correlation were used to compare mineral contents. Results: The hooves of both breeds were healthy and solid. The hooves of HA horses were longer than those of AA horses (14.90 ±0.30 cm vs 13.10 ±0.60 cm), while the AA hoof was harder than the HA hoof both in the wall (74.55 ±2.95 H vs 60.18 ±2.67 H) and sole (67.00 ±5.87 H vs 43.0 ±4.76 H). In comparison with the sole, the AA hoof wall also had a lower moisture percentage (12.56 ±0.67% vs 20.64 ±0.76%), while crude protein and ash contents were similar in both regions. The AA hoof showed a higher Se content, while the HA hoof had a higher level of macroelements. -
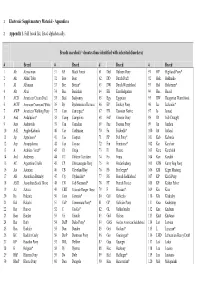
Electronic Supplementary Material - Appendices
1 Electronic Supplementary Material - Appendices 2 Appendix 1. Full breed list, listed alphabetically. Breeds searched (* denotes those identified with inherited disorders) # Breed # Breed # Breed # Breed 1 Ab Abyssinian 31 BF Black Forest 61 Dul Dülmen Pony 91 HP Highland Pony* 2 Ak Akhal Teke 32 Boe Boer 62 DD Dutch Draft 92 Hok Hokkaido 3 Al Albanian 33 Bre Breton* 63 DW Dutch Warmblood 93 Hol Holsteiner* 4 Alt Altai 34 Buc Buckskin 64 EB East Bulgarian 94 Huc Hucul 5 ACD American Cream Draft 35 Bud Budyonny 65 Egy Egyptian 95 HW Hungarian Warmblood 6 ACW American Creme and White 36 By Byelorussian Harness 66 EP Eriskay Pony 96 Ice Icelandic* 7 AWP American Walking Pony 37 Cam Camargue* 67 EN Estonian Native 97 Io Iomud 8 And Andalusian* 38 Camp Campolina 68 ExP Exmoor Pony 98 ID Irish Draught 9 Anv Andravida 39 Can Canadian 69 Fae Faeroes Pony 99 Jin Jinzhou 10 A-K Anglo-Kabarda 40 Car Carthusian 70 Fa Falabella* 100 Jut Jutland 11 Ap Appaloosa* 41 Cas Caspian 71 FP Fell Pony* 101 Kab Kabarda 12 Arp Araappaloosa 42 Cay Cayuse 72 Fin Finnhorse* 102 Kar Karabair 13 A Arabian / Arab* 43 Ch Cheju 73 Fl Fleuve 103 Kara Karabakh 14 Ard Ardennes 44 CC Chilean Corralero 74 Fo Fouta 104 Kaz Kazakh 15 AC Argentine Criollo 45 CP Chincoteague Pony 75 Fr Frederiksborg 105 KPB Kerry Bog Pony 16 Ast Asturian 46 CB Cleveland Bay 76 Fb Freiberger* 106 KM Kiger Mustang 17 AB Australian Brumby 47 Cly Clydesdale* 77 FS French Saddlebred 107 KP Kirdi Pony 18 ASH Australian Stock Horse 48 CN Cob Normand* 78 FT French Trotter 108 KF Kisber Felver 19 Az Azteca -

Multidisciplinary Approach to Genetic Variability, Inbreeding and Fertility in the Endangered Sorraia Horse Breed
UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS Multidisciplinary approach to genetic variability, inbreeding and fertility in the endangered Sorraia horse breed Doutoramento em Biologia Biologia da Conservação Helena Josefina Kjöllerström Tese orientada por: Profª Doutora Maria do Mar Jácome Félix Oom Prof. Doutor Bhanu Pratap Chowdhary Documento especialmente elaborado para a obtenção do grau de doutor 2016 UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS Multidisciplinary approach to genetic variability, inbreeding and fertility in the endangered Sorraia horse breed Doutoramento em Biologia Biologia da Conservação Helena Josefina Kjöllerström Tese orientada por: Profª Doutora Maria do Mar Jácome Félix Oom Prof. Doutor Bhanu Pratap Chowdhary Júri: Presidente: ●Doutora Maria Manuela Gomes Coelho de Noronha Trancoso Vogais: ● Doutora Terje Raudsepp ● Doutora Raquel Maria Garcia dos Santos Chaves ● Doutor José António dos Santos Pereira de Matos ● Doutor Luís Lavadinho Telo da Gama ● Doutora Maria Manuela Gomes Coelho de Noronha Trancoso ● Doutora Maria do Mar Jácome Félix Oom Documento especialmente elaborado para a obtenção do grau de doutor SFRH/BD/81502/2011, cE3c UID/BIA/00329/2013, PRODER (57882)(EU)/Action 2.2.3 (2011-2013), LINK Equine Research Endowment, American Quarter Horse Foundation and USDA (#2012-67015-19632) 2016 This work was supported by FCT/MEC (Fundação para a Ciência e Tecnologia) PhD Fellowship (SFRH/BD/81502/2011), cE3c FCT Unit funding (Ref. UID/BIA/00329/2013), PRODER (Contract number 57882)(EU)/Action 2.2.3 (2011-2013) [Conservation and improvement of genetic resources, Sub-action 2.2.3.2. Animal component (Sorraia breed)], LINK Equine Research Endowment, American Quarter Horse Foundation and USDA (grant #2012-67015-19632). -

TARPAN Or KONIK.Rtf
TARPAN OR KONIK ? An analysis of “semantic denaturation” The horse which is in the process of becoming de-domesticated and recovering little by little its place in some European ecosystems, especially in the Netherlands, often comes under the name of the Konik (or Konik Polski).This name, imported from Poland, is used inasmuch as the name Tarpan is considered to be applicable to the wild horse which reputedly ceased to exist in 1879. This deliberately limiting choice fails to take into account a certain number of scientific and historical facts. Moreover, it maintains an erroneous perception of the horse with the public which in general finds it hard to imagine that a horse can leave the domestic arena. The descendant of the wild horse which became the little Polish horse After the discovery of descendants of the tarpan with farmers of the Bilgoraj region at the beginning of the 20th century, Professor Tadeusz Vetulani undertook to save this primitive strain. His aim was to get back to the wild tarpan and introduce it, like the European bison, into its last-known refuge: the Bialowieza Forest. When Vetulani died in 1952, the dominant influence of some horse specialists ended up by consigning this horse to the traditional horse world, by giving it the official name of Konik Polski, literally "little Polish horse". Hence a genuine universal zoological heritage was relegated to the rank of a mere national breed of horse. Incidentally, it should be mentioned that if this horse is not specifically Polish (as is the case of the European bison), it is not "little" either. -

The Spanish Mustang and the Long Way Home by Callie Heacock and Ernesto Valdés
The Spanish Mustang and the Long Way Home by Callie Heacock and Ernesto Valdés The evolutionary history and preservation of the Spanish the runner of aboriginal wildness, I had to trace the Age of Horse Mustang is complex; its historical importance to the Spanish- Culture that he brought not only to Western tribes but to white Mexican settlements of Texas and, ultimately, to the colonization men who took their ranges. My chief pleasure has been in telling of the American West, cannot be overstated. J. Frank Dobie, who the tales, legendary as well as factual, of Mustangs and of rides spent years researching The Mustangs and is credited with the on horses of the Mustang breed—but historical business had to best chronicles of the horses ever written, estimated that, at their come before pleasure.”2 The Mustang history in the Americas is height, over a million Mustangs ran free in Texas. In The Mus- believed to begin with the arrival of the first Europeans; how- tangs, he wrote: “To comprehend the stallions that bore conquis- ever, an intriguing twist in its evolutionary path reveals that for tadores across the Americas, I had to go back to mares beside the horses, it was a homecoming. black tents in Arabian deserts. Before I could release myself with In 1493, on Christopher Columbus’ second voyage, twenty 16 Volume 7 • Number 1 • Fall 2009 Spanish horses stepped off the ships onto the Caribbean island to the Americas. As a result, historians cited the arrival of the of Santo Domingo and within a decade, this small band had horse with Columbus as the introduction of a new species into multiplied to over sixty horses. -

Posicionamiento Genético De La Raza Equina Hispano- Bretón
Actas Iberoamericanas de Conservación Animal AICA 5 (2015) 70-77 POSICIONAMIENTO GENÉTICO DE LA RAZA EQUINA HISPANO- BRETÓN GENETIC ANALYSIS OF THE HISPANO-BRETON HORSE BREED Cortés O.1*, Vega-Pla J.L.2, Berruezo A.3, Chomon N.4, Oom M.M.5, Dunner S.1, Delgado J.V.6, Gama L.7, Ginja C.5, Jordana J.8, Landi V.6,9, Luís C.5,10, Martín-Burriel I.11, Martínez A.M.6,9, Zaragoza P.11, Cañón J.1, BioHorse Consortium12 1Departamento de Producción Animal. Facultad de Veterinaria. Universidad Complutense de Madrid. Madrid. España. *[email protected] 2Laboratorio de Investigación Aplicada. Ministerio de Defensa. España. 3Consejería de Desarrollo Rural, Ganadería, Pesca y Biodiversidad. Santander. España. 4Centro de Selección y Reproducción Animal (CENSYRA). Cantabria. 5CE3C – Centre for Ecology, Evolution and Environmental Changes, Faculdade de Ciências, Universidade de Lisboa, 1749-016 Lisboa, Portugal. 6Universidad de Córdoba, España. 7CIISA – Faculdade de Medicina Veterinária, Universidade de Lisboa, Portugal. 8Universidad Autónoma de Barcelona, España. 9Animal Breeding Consulting S.L. España. 10Centro Interuniversitário de História das Ciências e da Tecnologia, Faculdade de Ciências, Universidade de Lisboa, Portugal. 11Universidad de Zaragoza, España. 12BioHorse Consortium: http://biohorse.jimdo.com/ Keywords: Hispano-Breton Abstract horse breed The Hispano-Breton equine breed is located in the North of the Iberian Peninsula Genetic diversity and currently is an endangered breed. In order to analyze the genetic variability of Genetic the Hispano-Breton horse breed and its genetic relationships with other horse breeds relationship located in the Iberian Peninsula a total of 25 autosomal microsatellites have been Molecular markers analyzed in 30 samples of the Hispano-Breton horse breed and in an additional 20 Iberian horse horse breeds that represent a comprehensive sampling of current Iberian Peninsula breeds horse breeds. -

Complaint Report
EXHIBIT A ARKANSAS LIVESTOCK & POULTRY COMMISSION #1 NATURAL RESOURCES DR. LITTLE ROCK, AR 72205 501-907-2400 Complaint Report Type of Complaint Received By Date Assigned To COMPLAINANT PREMISES VISITED/SUSPECTED VIOLATOR Name Name Address Address City City Phone Phone Inspector/Investigator's Findings: Signed Date Return to Heath Harris, Field Supervisor DP-7/DP-46 SPECIAL MATERIALS & MARKETPLACE SAMPLE REPORT ARKANSAS STATE PLANT BOARD Pesticide Division #1 Natural Resources Drive Little Rock, Arkansas 72205 Insp. # Case # Lab # DATE: Sampled: Received: Reported: Sampled At Address GPS Coordinates: N W This block to be used for Marketplace Samples only Manufacturer Address City/State/Zip Brand Name: EPA Reg. #: EPA Est. #: Lot #: Container Type: # on Hand Wt./Size #Sampled Circle appropriate description: [Non-Slurry Liquid] [Slurry Liquid] [Dust] [Granular] [Other] Other Sample Soil Vegetation (describe) Description: (Place check in Water Clothing (describe) appropriate square) Use Dilution Other (describe) Formulation Dilution Rate as mixed Analysis Requested: (Use common pesticide name) Guarantee in Tank (if use dilution) Chain of Custody Date Received by (Received for Lab) Inspector Name Inspector (Print) Signature Check box if Dealer desires copy of completed analysis 9 ARKANSAS LIVESTOCK AND POULTRY COMMISSION #1 Natural Resources Drive Little Rock, Arkansas 72205 (501) 225-1598 REPORT ON FLEA MARKETS OR SALES CHECKED Poultry to be tested for pullorum typhoid are: exotic chickens, upland birds (chickens, pheasants, pea fowl, and backyard chickens). Must be identified with a leg band, wing band, or tattoo. Exemptions are those from a certified free NPIP flock or 90-day certificate test for pullorum typhoid. Water fowl need not test for pullorum typhoid unless they originate from out of state. -

Decreased Genetic Diversity in Kiso Horses Revealed Through Annual Microsatellite Genotyping
Advance Publication The Journal of Veterinary Medical Science Accepted Date: 20 February 2020 J-STAGE Advance Published Date: 9 March 2020 ©2020 The Japanese Society of Veterinary Science Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. 1 2 Note 3 Wildlife Science 4 5 6 Decreased genetic diversity in Kiso horses revealed through annual microsatellite genotyping 7 8 Authors: Mako NAKAMURA,1 Teruaki TOZAKI,1,2 Hironaga KAKOI,2 Kotono NAKAMURA,1 Reza 9 RAJABI-TOUSTANI,1 Yasunori OHBA,1, 3 Tatsuya MATSUBARA,1 and Masaki TAKASU1, 3 10 11 1 Department of Veterinary Medicine, Faculty of Applied Biological Sciences, Gifu University, 1-1 Yanagido, 12 Gifu, 501-1193, Japan 13 2 Laboratory of Racing Chemistry, 1731-2 Tsurutamachi, Utsunomiya, Tochigi, 320-0851, Japan 14 3 Education and Research Center for Food Animal Health (GeFAH), Gifu University, 1-1 Yanagido, Gifu, 15 501-1193, Japan 16 17 Running Head: DECREASED GENETIC DIVERSITY IN KISO HORSES 18 19 Correspondence: Masaki Takasu, Department of Veterinary Medicine, Faculty of Applied Biological Sciences, 20 Gifu University, 1-1 Yanagido, Gifu 501-1193, Japan 21 Phone: +81-58-293-2955; Email: [email protected] 22 23 24 1 25 ABSTRACT 26 The Kiso horse is native to Japan and is on the verge of extinction. Here, we used microsatellites 27 to characterize changes in their genetic diversity over time. We divided a population of Kiso 28 horses that genotyped during 2007-2017 into three groups based on birth year: Group 1, 29 1980–1998 (70 horses); Group 2, 1999–2007 (61 horses); and Group 3, 2008–2017 (42 horses). -

Horses As Sources of Proprietary Information: Commercialization, Conservation, and Compensation Pursuant to the Convention on Biological Diversity
University of New Hampshire University of New Hampshire Scholars' Repository University of New Hampshire – Franklin Pierce Law Faculty Scholarship School of Law 1-1-2014 Horses as Sources of Proprietary Information: Commercialization, Conservation, and Compensation Pursuant to the Convention on Biological Diversity Haley McClory University of New Hampshire School of Law Stanley Kowalski University of New Hampshire School of Law, [email protected] Follow this and additional works at: https://scholars.unh.edu/law_facpub Part of the Animal Sciences Commons, Animal Studies Commons, and the Genetics and Genomics Commons Recommended Citation Haley McClory & Stanley P. Kowalski, "Horses as Sources of Proprietary Information: Commercialization, Conservation, and Compensation Pursuant to the Convention on Biological Diversity," 17 AGBIOFORUM, 141 (2014), available at http://perma.cc/S2FY-SPJB This Article is brought to you for free and open access by the University of New Hampshire – Franklin Pierce School of Law at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Law Faculty Scholarship by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. AgBioForum, 17(2): 141-155. ©2014 AgBioForum. Horses as Sources of Proprietary Information: Commercialization, Conservation, and Compensation Pursuant to the Convention on Biological Diversity Haley McClory and Stanley P. Kowalski Horses indigenous to East and Southeast (E/SE) Asia, including University of New Hampshire, School of Law, International native, landrace, feral, and wild populations, embody valuable Technology Transfer Institute (ITTI) genetic diversity. Conservation efforts for animals have largely been driven by humane altruism, with little consideration for the information value of genomes.