Endophytic Bacterial Communities Associated with Roots and Leaves of Plants Growing in Chilean Extreme Environments
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Plantings in the Arboretum the YEAR in REVIEW
Four new have been Four new Yoshino cherry trees have Yoshinobeen planted along planted Azalea Way. cherry trees along Azalea New Plantings in the Arboretum THE YEAR IN REVIEW T EX T B Y R AY L A R SON P HO T OS B Y N IA ll D UNNE n the five years that I have been curator, 2018 was the most active in terms of new plantings in the Arboretum. A majority of these centered around the new Arboretum Loop Trail and adjacent areas, many of which were enhanced, rehabilitated and Iaugmented. We also made improvements to a few other collection and garden areas with individual and smaller plantings. Following is a summary of some of the more noticeable new plantings you might encounter during your next visit. Winter 2019 v 3 Arboretum Entrance Perhaps the most obvious major planting occurred in March, just north of the Graham Visitors Center, with the creation of a new, large bed at the southeast corner of the intersection of Arboretum Drive and Foster Island Road. This intersection changed a lot as part of the Loop Trail construction—with the addition of new curbs and crosswalks—and we wanted to create a fitting entrance to the Arboretum at its north end. The new planting was also intended to alleviate some of the soil compaction and social trails that had developed on the east side of Arboretum Drive during trail construction. What’s more, we wanted to encourage pedes- trians to use the new gravel trail on the west side of the Drive to connect from the lower parking lots to the Visitors Center—rather than walk in the road. -

Planting Schemes Advice Note 2021
Natural Environment Team East Dorset Environment Partnership Dorset Biodiversity Appraisal Protocol Advice Note Planting scheme recommendations Introduction This advice note was written with the East Dorset Environment Partnership and is intended primarily to assist ecological consultants and developers when submitting Biodiversity Plans (BPs) and Landscape & Ecological Management Plans (LEMPs) to DC NET for review under the Dorset Biodiversity Appraisal Protocol (DBAP) by describing how to maximise the biodiversity potential of good planting schemes designed to deliver multiple benefits and contribute to achieving biodiversity net gain. Making the most of existing habitats strengthened through strong eco-tones; sound planting composition; connectivity to ecological networks within and beyond site boundaries and appropriate on-going management are all fundamental elements of an outstanding planting scheme. Submitted planting schemes for developments should seek to offer biodiversity benefit and comply with Dorset Council’s Pollinators Action Plan and Green Infrastructure Strategies. Schemes should demonstrate how they will contribute to addressing the Climate & Ecological Emergency Strategy (Draft 2020). Currently, many schemes appear to be generic designs that do not take account of local conditions and are based on widely available and low-cost shrubs; many of which are invasive, potentially invasive or nuisance plants known as ‘garden thugs’. This is of particular concern where new sites for development are on the rural fringe and pose a significant risk of spreading damaging alien plant species into the wider countryside and sensitive semi-natural habitats. Recent published work by the Royal Horticultural Society (RHS) and others has focussed on lists of plants that attract pollinators rather than broader biodiversity considerations. -

WO 2018/064165 A2 (.Pdf)
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/064165 A2 05 April 2018 (05.04.2018) W !P O PCT (51) International Patent Classification: Published: A61K 35/74 (20 15.0 1) C12N 1/21 (2006 .01) — without international search report and to be republished (21) International Application Number: upon receipt of that report (Rule 48.2(g)) PCT/US2017/053717 — with sequence listing part of description (Rule 5.2(a)) (22) International Filing Date: 27 September 2017 (27.09.2017) (25) Filing Language: English (26) Publication Langi English (30) Priority Data: 62/400,372 27 September 2016 (27.09.2016) US 62/508,885 19 May 2017 (19.05.2017) US 62/557,566 12 September 2017 (12.09.2017) US (71) Applicant: BOARD OF REGENTS, THE UNIVERSI¬ TY OF TEXAS SYSTEM [US/US]; 210 West 7th St., Austin, TX 78701 (US). (72) Inventors: WARGO, Jennifer; 1814 Bissonnet St., Hous ton, TX 77005 (US). GOPALAKRISHNAN, Vanch- eswaran; 7900 Cambridge, Apt. 10-lb, Houston, TX 77054 (US). (74) Agent: BYRD, Marshall, P.; Parker Highlander PLLC, 1120 S. Capital Of Texas Highway, Bldg. One, Suite 200, Austin, TX 78746 (US). (81) Designated States (unless otherwise indicated, for every kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Table S5. the Information of the Bacteria Annotated in the Soil Community at Species Level
Table S5. The information of the bacteria annotated in the soil community at species level No. Phylum Class Order Family Genus Species The number of contigs Abundance(%) 1 Firmicutes Bacilli Bacillales Bacillaceae Bacillus Bacillus cereus 1749 5.145782459 2 Bacteroidetes Cytophagia Cytophagales Hymenobacteraceae Hymenobacter Hymenobacter sedentarius 1538 4.52499338 3 Gemmatimonadetes Gemmatimonadetes Gemmatimonadales Gemmatimonadaceae Gemmatirosa Gemmatirosa kalamazoonesis 1020 3.000970902 4 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sphingomonas indica 797 2.344876284 5 Firmicutes Bacilli Lactobacillales Streptococcaceae Lactococcus Lactococcus piscium 542 1.594633558 6 Actinobacteria Thermoleophilia Solirubrobacterales Conexibacteraceae Conexibacter Conexibacter woesei 471 1.385742446 7 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sphingomonas taxi 430 1.265115184 8 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sphingomonas wittichii 388 1.141545794 9 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sphingomonas sp. FARSPH 298 0.876754244 10 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sorangium cellulosum 260 0.764953367 11 Proteobacteria Deltaproteobacteria Myxococcales Polyangiaceae Sorangium Sphingomonas sp. Cra20 260 0.764953367 12 Proteobacteria Alphaproteobacteria Sphingomonadales Sphingomonadaceae Sphingomonas Sphingomonas panacis 252 0.741416341 -

Exploring the Diversity and Antimicrobial Potential of Marine Actinobacteria from the Comau Fjord in Northern Patagonia, Chile
ORIGINAL RESEARCH published: 19 July 2016 doi: 10.3389/fmicb.2016.01135 Exploring the Diversity and Antimicrobial Potential of Marine Actinobacteria from the Comau Fjord in Northern Patagonia, Chile Agustina Undabarrena 1, Fabrizio Beltrametti 2, Fernanda P. Claverías 1, Myriam González 1, Edward R. B. Moore 3, 4, Michael Seeger 1 and Beatriz Cámara 1* 1 Laboratorio de Microbiología Molecular y Biotecnología Ambiental, Departamento de Química & Centro de Biotecnología Daniel Alkalay Lowitt, Universidad Técnica Federico Santa María, Valparaíso, Chile, 2 Actygea S.r.l., Gerenzano, Italy, 3 Culture Collection University of Gothenburg (CCUG), Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden, 4 Department of Infectious Diseases, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden Edited by: Bioprospecting natural products in marine bacteria from fjord environments are attractive Learn-Han Lee, due to their unique geographical features. Although, Actinobacteria are well known Monash University Malaysia Campus, Malaysia for producing a myriad of bioactive compounds, investigations regarding fjord-derived Reviewed by: marine Actinobacteria are scarce. In this study, the diversity and biotechnological Atte Von Wright, potential of Actinobacteria isolated from marine sediments within the Comau University of Eastern Finland, Finland fjord, in Northern Chilean Patagonia, were assessed by culture-based approaches. Polpass Arul Jose, Central Salt and Marine Chemicals The 16S rRNA gene sequences revealed that members phylogenetically related Research Institute, India to the Micrococcaceae, Dermabacteraceae, Brevibacteriaceae, Corynebacteriaceae, *Correspondence: Microbacteriaceae, Dietziaceae, Nocardiaceae, and Streptomycetaceae families were Beatriz Cámara [email protected] present at the Comau fjord. A high diversity of cultivable Actinobacteria (10 genera) was retrieved by using only five different isolation media. -

Gardenergardener®
Theh American A n GARDENERGARDENER® The Magazine of the AAmerican Horticultural Societyy January / February 2016 New Plants for 2016 Broadleaved Evergreens for Small Gardens The Dwarf Tomato Project Grow Your Own Gourmet Mushrooms contents Volume 95, Number 1 . January / February 2016 FEATURES DEPARTMENTS 5 NOTES FROM RIVER FARM 6 MEMBERS’ FORUM 8 NEWS FROM THE AHS 2016 Seed Exchange catalog now available, upcoming travel destinations, registration open for America in Bloom beautifi cation contest, 70th annual Colonial Williamsburg Garden Symposium in April. 11 AHS MEMBERS MAKING A DIFFERENCE Dale Sievert. 40 HOMEGROWN HARVEST Love those leeks! page 400 42 GARDEN SOLUTIONS Understanding mycorrhizal fungi. BOOK REVIEWS page 18 44 The Seed Garden and Rescuing Eden. Special focus: Wild 12 NEW PLANTS FOR 2016 BY CHARLOTTE GERMANE gardening. From annuals and perennials to shrubs, vines, and vegetables, see which of this year’s introductions are worth trying in your garden. 46 GARDENER’S NOTEBOOK Link discovered between soil fungi and monarch 18 THE DWARF TOMATO PROJECT BY CRAIG LEHOULLIER butterfl y health, stinky A worldwide collaborative breeds diminutive plants that produce seeds trick dung beetles into dispersal role, regular-size, fl avorful tomatoes. Mt. Cuba tickseed trial results, researchers unravel how plants can survive extreme drought, grant for nascent public garden in 24 BEST SMALL BROADLEAVED EVERGREENS Delaware, Lady Bird Johnson Wildfl ower BY ANDREW BUNTING Center selects new president and CEO. These small to mid-size selections make a big impact in modest landscapes. 50 GREEN GARAGE Seed-starting products. 30 WEESIE SMITH BY ALLEN BUSH 52 TRAVELER’S GUIDE TO GARDENS Alabama gardener Weesie Smith championed pagepage 3030 Quarryhill Botanical Garden, California. -

Perennials for Winter Gardens Perennials for Winter Gardens
TheThe AmericanAmerican GARDENERGARDENER® TheThe MagazineMagazine ofof thethe AAmericanmerican HorticulturalHorticultural SocietySociety November / December 2010 Perennials for Winter Gardens Edible Landscaping for Small Spaces A New Perspective on Garden Cleanup Outstanding Conifers contents Volume 89, Number 6 . November / December 2010 FEATURES DEPARTMENTS 5 NOTES FROM RIVER FARM 6 MEMBERS’ FORUM 8 NEWS FROM THE AHS Boston’s garden contest grows to record size, 2011 AHS President’s Council trip planned for Houston, Gala highlights, rave reviews for Armitage webinar in October, author of article for The American Gardener receives garden-writing award, new butterfly-themed children’s garden installed at River Farm. 12 2010 AMERICA IN BLOOM AWARD WINNERS Twelve cities are recognized for their community beautification efforts. 42 ONE ON ONE WITH… David Karp: Fruit detective. page 26 44 HOMEGROWN HARVEST The pleasures of popcorn. EDIBLE LANDSCAPING FOR SMALL SPACES 46 GARDENER’S NOTEBOOK 14 Replacing pavement with plants in San BY ROSALIND CREASY Francisco, soil bacterium may boost cognitive With some know-how, you can grow all sorts of vegetables, fruits, function, study finds fewer plant species on and herbs in small spaces. earth now than before, a fungus-and-virus combination may cause honeybee colony collapse disorder, USDA funds school garden CAREFREE MOSS BY CAROLE OTTESEN 20 program, Park Seed sold, Rudbeckia Denver Looking for an attractive substitute for grass in a shady spot? Try Daisy™ wins grand prize in American moss; it’ll grow on you. Garden Award Contest. 50 GREEN GARAGE® OUTSTANDING CONIFERS BY RITA PELCZAR 26 A miscellany of useful garden helpers. This group of trees and shrubs is beautiful year round, but shines brightest in winter. -

Mapping the Bacterial Ecology on the Phyllosphere Of
bioRxiv preprint doi: https://doi.org/10.1101/494799; this version posted December 12, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Mapping the bacterial ecology on the phyllosphere of grass hay and the 2 potential hazards of soaking fodder for horse gut health 3 Meriel JS Moore-Colyer1, Annette Longland2, Patricia Harris3, Susan Crosthwaite4 4 1 Royal Agricultural University, Cirencester Gloucestershire, UK GL7 6JS. 5 2Equine and Livestock Nutrition Services, Pantafallen Fach, Tregaron, Ceredigion, Wales. SY25 6NF 6 3Mars Horsecare UK LTD; Equine Studies Group, Waltham Centre for Pet Nutrition, Waltham-on-the Wolds, 7 Leicestershire, UK. LE14 4RT 8 4 Faculty of Biology, Medicine and Health, University of Manchester, UK M13 9PT. 9 10 Corresponding author: *[email protected] ORCID 0000-0002-9172-9862 11 12 Short title: The bacterial ecology on the phyllosphere of dry and post-soaked grass hay for 13 horses 14 bioRxiv preprint doi: https://doi.org/10.1101/494799; this version posted December 12, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 15 Abstract 16 Globally hay is the preferred forage for stabled horses. Variable nutritional and hygienic quality stimulates pre- 17 feeding soaking to reduce dust and nutrients to reduce respiratory and metabolic disorders in horses. -

Marine Rare Actinomycetes: a Promising Source of Structurally Diverse and Unique Novel Natural Products
Review Marine Rare Actinomycetes: A Promising Source of Structurally Diverse and Unique Novel Natural Products Ramesh Subramani 1 and Detmer Sipkema 2,* 1 School of Biological and Chemical Sciences, Faculty of Science, Technology & Environment, The University of the South Pacific, Laucala Campus, Private Mail Bag, Suva, Republic of Fiji; [email protected] 2 Laboratory of Microbiology, Wageningen University & Research, Stippeneng 4, 6708 WE Wageningen, The Netherlands * Correspondence: [email protected]; Tel.: +31-317-483113 Received: 7 March 2019; Accepted: 23 April 2019; Published: 26 April 2019 Abstract: Rare actinomycetes are prolific in the marine environment; however, knowledge about their diversity, distribution and biochemistry is limited. Marine rare actinomycetes represent a rather untapped source of chemically diverse secondary metabolites and novel bioactive compounds. In this review, we aim to summarize the present knowledge on the isolation, diversity, distribution and natural product discovery of marine rare actinomycetes reported from mid-2013 to 2017. A total of 97 new species, representing 9 novel genera and belonging to 27 families of marine rare actinomycetes have been reported, with the highest numbers of novel isolates from the families Pseudonocardiaceae, Demequinaceae, Micromonosporaceae and Nocardioidaceae. Additionally, this study reviewed 167 new bioactive compounds produced by 58 different rare actinomycete species representing 24 genera. Most of the compounds produced by the marine rare actinomycetes present antibacterial, antifungal, antiparasitic, anticancer or antimalarial activities. The highest numbers of natural products were derived from the genera Nocardiopsis, Micromonospora, Salinispora and Pseudonocardia. Members of the genus Micromonospora were revealed to be the richest source of chemically diverse and unique bioactive natural products. -
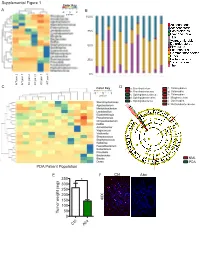
Mental Figure 1 Color Key a -2 0 2 B Z-Score 100%
Supplemental Figure 1 Color Key A -2 0 2 B z-score 100% 75% 50% 25% 0% KC pan 1 WT pan 3 WT KC pan 3 WT pan 2 WT pan 1 WT KC pan 2 C Color Key D a: Brevibacterium f: Chlamydiales b: Brevibacteriaceae g: Chlamydiia -3 0 3 z-score c: Sphingobacteriaceae h: Chlamydiae d: Sphingobacteriales i: Mogibacterium e: Sphingobacteriia j: Oscillospira k: Methylobacteriaceae NML Control Microb.-entrained MΦ PDA PDA Patient Population Control Microb.-entrained MΦ + Myd88i E F Ctrl Abx 350 * 300 250 200 150 40X 100 Tumor weight (mg) 50 0 x Ctrl Ab Supplemental Figure 2 A KC WT B ** * Actinobacteria * ** Bacteroidetes Cyanobacteria Deferribacteres * Firmicutes Proteobacteria % Relative abundance TM7 Others Time(wks) 3 9 13 16 20 24 28 32 36 3 9 13 16 20 24 28 32 36 Alpha Diversity Measure C E 60 KC WT 40 20 B. pseudolongum B. animalis 60 5 KC WT 0 B. adolescentis 40% Rel. abundance 3 9 13 16 20 24 28 32 36 3 9 13 16 20 24 28 32 36 20 Age (weeks) B. pseudolongum B. animalis 5 0 B. adolescentis % Rel. abundance 3 9 13 16 20 24 28 32 36 3 9 13 16 20 24 28 32 36 F Age (weeks) Week 3 Week 9 Week 13 p=0.678 p=0.02 p=0.385 Time(wks) 3 9 24 20 13 16 D 28 32 36 Week 13 KC WT Firmicutes; Ruminococcus Firmicutes; Dehalobacterium Alpha Diversity Measure Firmicutes; Oscillospira Bacteroidates; Odoribacter Axis.2 [12.7%] Actinobacteria; Bifidobacterium Axis.2 [23.8%] Axis.2 [24.7%] Week 16 Bacteroidetes; Bacteroidales Axis.1 [80.8%] Axis.1 [65.4%] Axis.1 [49.6%] Actinobacteria; Bifidobacterium Week 16 Week 20 Week 24 Week 20 p=0.339 p=0.036 p=0.021 Firmicutes; Dehalobacterium -

1 Supplementary Material a Major Clade of Prokaryotes with Ancient
Supplementary Material A major clade of prokaryotes with ancient adaptations to life on land Fabia U. Battistuzzi and S. Blair Hedges Data assembly and phylogenetic analyses Protein data set: Amino acid sequences of 25 protein-coding genes (“proteins”) were concatenated in an alignment of 18,586 amino acid sites and 283 species. These proteins included: 15 ribosomal proteins (RPL1, 2, 3, 5, 6, 11, 13, 16; RPS2, 3, 4, 5, 7, 9, 11), four genes (RNA polymerase alpha, beta, and gamma subunits, Transcription antitermination factor NusG) from the functional category of Transcription, three proteins (Elongation factor G, Elongation factor Tu, Translation initiation factor IF2) of the Translation, Ribosomal Structure and Biogenesis functional category, one protein (DNA polymerase III, beta subunit) of the DNA Replication, Recombination and repair category, one protein (Preprotein translocase SecY) of the Cell Motility and Secretion category, and one protein (O-sialoglycoprotein endopeptidase) of the Posttranslational Modification, Protein Turnover, Chaperones category, as annotated in the Cluster of Orthologous Groups (COG) (Tatusov et al. 2001). After removal of multiple strains of the same species, GBlocks 0.91b (Castresana 2000) was applied to each protein in the concatenation to delete poorly aligned sites (i.e., sites with gaps in more than 50% of the species and conserved in less than 50% of the species) with the following parameters: minimum number of sequences for a conserved position: 110, minimum number of sequences for a flank position: 110, maximum number of contiguous non-conserved positions: 32000, allowed gap positions: with half. The signal-to-noise ratio was determined by altering the “minimum length of a block” parameter. -

Phytophoto Index 2018
PhytoPhoto 2018 Image Availability Accessing the photo collection is easy. Simply send an email with the plant names or a description of images sought to [email protected] and a gallery of photos meeting your criteria will be submitted to you, usually the same day. Abeliophyllum distichum Abutilon vitifolium ‘Album’ Acer palmatum fall color Abeliophyllum distichum ‘Roseum’ Abutilon vitifolium white Acer palmatum in front of window Abelmoschus esculentus "Okra" Abutilon Wisley Red Acer palmatum in orange fall color Abelmoschus manihot Abutilon x hybridum 'Bella Red' Acer palmatum var. dissectum Abies balsamea 'Nana' Abutilon-orange Acer palmatum var. dissectum Dissectum Abies concolor 'Blue Cloak' Abutilon-white Viride Group Abies guatemalensis Acacia baileyana Acer pensylvaticum Abies koreana 'Glauca' Acacia baileyana 'Purpurea' Acer platanoides 'Princeton Gold' Abies koreana 'Green Carpet' Acacia boormanii Acer pseudoplatanus Abies koreana 'Horstmann's Silberlocke' Acacia confusa Acer pseudoplatanus 'Leopoldii' Abies koreana 'Silberperle' Acacia cultriformis Acer pseudoplatanus 'Purpureum' Abies koreana 'Silberzwerg' Acacia dealbata Acer pseudoplatanus ‘Puget Pink’ Abies koreana 'Silver Show' Acacia iteaphylla Acer pseudoplatanus f... 'Leopoldii' Abies koreana Aurea Acacia koa Acer rubrum Abies koreana-cone Acacia koa seedlings Acer rubrum and stop sign Abies lasiocarpa Acacia koaia Acer rufinerve Hatsuyuki Abies lasiocarpa v. arizonica 'Argentea' Acacia longifolia Acer saccharinum Abies lasiocarpa v. arizonica 'Glauca Acacia