M. Phil Psycho-Oncology (Affiliated to the University of Madras)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Global Retinoblastoma Presentation and Analysis by National Income Level
Research JAMA Oncology | Original Investigation Global Retinoblastoma Presentation and Analysis by National Income Level Global Retinoblastoma Study Group Supplemental content IMPORTANCE Early diagnosis of retinoblastoma, the most common intraocular cancer, can save both a child’s life and vision. However, anecdotal evidence suggests that many children across the world are diagnosed late. To our knowledge, the clinical presentation of retinoblastoma has never been assessed on a global scale. OBJECTIVES To report the retinoblastoma stage at diagnosis in patients across the world during a single year, to investigate associations between clinical variables and national income level, and to investigate risk factors for advanced disease at diagnosis. DESIGN, SETTING, AND PARTICIPANTS A total of 278 retinoblastoma treatment centers were recruited from June 2017 through December 2018 to participate in a cross-sectional analysis of treatment-naive patients with retinoblastoma who were diagnosed in 2017. MAIN OUTCOMES AND MEASURES Age at presentation, proportion of familial history of retinoblastoma, and tumor stage and metastasis. RESULTS The cohort included 4351 new patients from 153 countries; the median age at diagnosis was 30.5 (interquartile range, 18.3-45.9) months, and 1976 patients (45.4%) were female. Most patients (n = 3685 [84.7%]) were from low- and middle-income countries (LMICs). Globally, the most common indication for referral was leukocoria (n = 2638 [62.8%]), followed by strabismus (n = 429 [10.2%]) and proptosis (n = 309 [7.4%]). Patients from high-income countries (HICs) were diagnosed at a median age of 14.1 months, with 656 of 666 (98.5%) patients having intraocular retinoblastoma and 2 (0.3%) having metastasis. -

2016-2017 Indian Institute of Technology Madras
Indian Institute of Technology Madras 2016-2017 No. 1 Engineering Institute in the Country for 2016, 2017 & 2018 As per National Institutional Ranking Framework, MHRD, Govt. of India CoNtents YEAR AT A GLANCE 2 DIRECTOR’S REPORT 4 ADMINISTRATION 20 ACADEMIC PROGRAMMES AND AWARD OF DEGREES 24 DEPARTMENTS CENTRES OF SPECIAL FACILITIES DEPARTMENT OF AEROSPACE ENGINEERING 34 CENTRE FOR INDUSTRIAL CONSULTANCY & SPONSORED RESEARCH 68 DEPARTMENT OF APPLIED MECHANICS 36 CENTRE FOR CONTINUING EDUCATION 70 DEPARTMENT OF BIOTECHNOLOgy 38 P.G. SENAPATHY CENTRE FOR COMPUTING RESOURCES 71 DEPARTMENT OF CHEMISTRY 40 CENTRAL ELECTRONICS CENTRE 72 DEPARTMENT OF CHEMICAL ENGINEERING 42 SOPHISTICATED ANALYTICAL INSTRUMENT FACILITY 73 DEPARTMENT OF CIVIL ENGINEERING 44 CENTRAL FACILITIES 74 DEPARTMENT OF COMPUTER SCIENCE AND ENGINEERING 46 CENTRAL LIBRARY 75 DEPARTMENT OF ELECTRICAL ENGINEERING 48 STUDENTS AMENITIES & ACTIVITIES 76 DEPARTMENT OF ENGINEERING DESIGN 50 INTERNATIONAL & ALUMNI RELATIONS 78 DEPARTMENT OF HUMANITIES AND SOCIAL SCIENCES 52 CENTRES OF EXCELLENCE 80 DEPARTMENT OF MANAGEMENT STUDIES 54 STUDENTS PLACEMENT 81 DEPARTMENT OF MATHEMATICS 56 FINANCIAL ASSISTANCE TO STUDENTS 82 DEPARTMENT OF MECHANICAL ENGINEERING 58 FINANCE & ACCOUNTS 84 DEPARTMENT OF METALLURGICAL AND MATERIALS ENGINEERING 60 CAMPUS AMENITIES 86 DEPARTMENT OF OCEAN ENGINEERING 62 DEPARTMENT OF PHYSICS 64 Year Book 2016–17 1 YEAR At A GlANCE UG Students on roll 1982 465 UG Admissions PG Students on roll 4431 1316 PG Admissions Research Scholars on roll 2767 494 Research -

CTRI Trial Data
PDF of Trial CTRI Website URL - http://ctri.nic.in Clinical Trial Details (PDF Generation Date :- Wed, 29 Sep 2021 03:01:46 GMT) CTRI Number CTRI/2009/091/000298 [Registered on: 16/07/2009] - Last Modified On 26/09/2014 Post Graduate Thesis No Type of Trial Interventional Type of Study Vaccine Other (Specify) [Pilot study to study the safety and immunogenicity of Merck Quadrivalent HPV vaccine] Study Design Single Arm Trial Public Title of Study A clinical trial to study prevention of HPV in HIV-Positive women in India Scientific Title of A Single-Arm, Open-Label, Pilot Study of the Safety and Immunogenicity of the Merck Quadrivalent Study Human Papillomavirus Vaccine among HIV-Positive Women in India: A Trial of AIDS Malignancy Clinical Trials Consortium Acronym: AMC Protocol # 054 Secondary IDs if Any Secondary ID Identifier AMC-054 Version 9.0; May 4 2012 Protocol Number NCT00667563 ClinicalTrials.gov Details of Principal Details of Principal Investigator Investigator or overall Name Dr N Kumarasamy Trial Coordinator (multi-center study) Designation Principal Investigator Affiliation YR Gaitonde Medical Educational & Research Foundation Address YR Gaitonde Medical Educational & Research Foundation, Voluntary Health Services, Rajiv Gandhi Salai, Taramani Chennai TAMIL NADU 600113 India Phone 04439106789 Fax 04422542949 Email [email protected] Details Contact Details Contact Person (Scientific Query) Person (Scientific Name Dr N Kumarasamy Query) Designation Principal Investigator Affiliation YR Gaitonde Medical Educational & Research Foundation Address YR Gaitonde Medical Educational & Research Foundation, Voluntary Health Services, Rajiv Gandhi Salai, Taramani Chennai TAMIL NADU 600113 India Phone 04439106789 Fax 04422542949 Email [email protected] Details Contact Details Contact Person (Public Query) Person (Public Query) Name Sumeela Nair Designation Lead Data Manager Affiliation The EMMES Corporation Address EMMES Services Pvt. -

Narrative Notes on Plan Programmes Tam-N
y » I ; ^ t O M i T*' NARRATIVE NOTES ON PLAN PROGRAMMES ANNUAL PLAN 2000-01 STATE PLANNING COMMISSION CHENNAI - 600 005 - 5 * 4 8 2 3 0 < » - 2 5 - TAM-N AUGUST 2000 NARRATIVE NOTES ON PLAN PROGRAMMES 2000-01 NIEPA DC D11079 ' xA^\Q§ i , , .‘♦1 Zi. i-I. Mr:,-, ' 3 )-u o 79 V ^ ' ' Z4* - o 4"* Zc © I CONTENTS Page 1. Crop Husbandry 1 2. Research and Education 25 3. Food, Storage & WareHousing 30 4. Soil & Water Conservation 35 5. Animal Husbandry 41 6. Dairy Developnnent 50 7. Fisheries 53 8. Forests 61 9. Investment in Agri.Financial Institutions 69 10. Co-operation 71 11. Special Programme for Rural Development 75 12. Land Reforms 79 13. Community Development 80 14. Minor Irrigation 83 15. Command Area Development 88 16. Major, Medium Irrigation & Flood Control 90 17. Power Development 103 18. Non-Conventional Sources of Energy 111 19. Industries- Medium and Large 114 20. Village and Small Industries 130 21. Weights and Measures 142 22. Mining and Metallurgical Industries 143 23. Roads and Bridges 145 24. Road and Inland Water Transport 156 25. Scientific Services and Research 158 26. Ecology and Environment 163 27. Secretariat Economic Services 166 28. Tourism 171 29. Economic Advice and Statistics 175 30. Civil Supplies 179 31. General Education 184 CONTENTS—conf. Pagee 32. Technical Education 1988 33. Art and Culture 2011 34. Sports and Youth Services 207)7 35. Medical 21C0 36. Public Health 2188 37. Water Supply and Sanitation 2332 38. Housing 24ft6 39. Urban Development 2551 40. Information and Publicity 2558 41. -

Government of India Ministry of Health and Family Welfare Department of Health and Family Welfare Lok Sabha Unstarred Question No
GOVERNMENT OF INDIA MINISTRY OF HEALTH AND FAMILY WELFARE DEPARTMENT OF HEALTH AND FAMILY WELFARE LOK SABHA UNSTARRED QUESTION NO. 559 TO BE ANSWERED ON 20th JULY, 2018 SETTING UP OF CANCER INSTITUTES 559. SHRI RAJIV PRATAP RUDY: DR. SHASHI THAROOR: SHRI K.C. VENUGOPAL: Will the Minister of HEALTH AND FAMILY WELFARE be pleased to state: (a) whether the Government proposes to set up more Cancer Institutes, Tertiary Care Cancer Centres (TCCCs), Regional Cancer Centres (RCC’s) in the different parts of the country; (b) if so, the details of the present and such proposed institutes/centres in the country; (c) whether the Government proposes to convert various RCCs into National Cancer Institutes, if so, the details thereof; (d) the details of proposal received by various State Governments to set up more such centres along with the action taken thereon; and (e) the steps taken by the Government to promote research projects and allocate funds for the same? ANSWER THE MINISTER OF STATE IN THE MINISTRY OF HEALTH AND FAMILY WELFARE (SMT. ANUPRIYA PATEL) (a) to (d): Under “Strengthening of Tertiary Care Cancer facilities” scheme of National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases and Stroke (NPCDCS), Government of India provides financial assistance for setting up of State Cancer Institutes (SCI) and Tertiary Care Cancer Centres (TCCC) in different parts of the country. The details of proposals received from various State Governments to set up SCIs and TCCCs is at Annexure-I and the details of SCIs and TCCCs approved and first installment of Government of India share released so far, is at Annexure-II. -

Central Leather Research Institute, Adyar, Chennai- 600020
CENTRAL LEATHER RESEARCH INSTITUTE, ADYAR, CHENNAI- 600020 LIST OF APPROVED HOSPITALS AND ITS BRANCHES FOR HEALTH SERVICES BY CLRI AS ON 01-04-2019 REIMBURSEMENT AS PER CGHS RATES ONLY Sl. No Name of the Hospitals Address 1. Amrit Hospital Mint Street, Sowcarpet, Chennai - 79 2. Adyar P.M. Hospital & Research Centre Pvt. Ltd Lattice Bridge Rd, Adyar, Chennai - 20 3. Andhra Mahila Sabha R.A.Puram, Chennai - 28 4. Apollo Speciality Hospital – OMR Perungudi, Chennai - 96 5. Apollo Hospitals Enterprises Greams Road, Chennai - 6 6. Apollo Multispeciality Hospitals Teynampet, Chennai - 18 7. Appasamy Medicare Centre (P) Ltd Arumbakkam, Chennai - 106 8. Aswine Soundra Hospital and Research Centre Teynampet, Chennai - 18 9. Athipathy Hospital & Research Centre Velachery, Chennai - 42 10. Balaji Hospitals Private Ltd Guindy, Chennai - 32 11. Balakrishna Eye Hospital & Eye Research Centre Saidapet, Chennai - 15 12. Barathiraja Hospital and Research Centre Pvt. T.Nagar, Chennai - 17 Ltd 13. Beach Chennai Hospital New Washermanpet, Chennai - 81 14. Billroth Hospital Shenoy Nagar, Chennai - 30 15. BSS Hospital Mandaveli, Chennai - 28 16. Cancer Institute (WIA) Adyar, Chennai - 20 17. Chettinad Health City Kelambakkam, Chennai-603103 18. Child Trust Hospital Nungambakkam, Chennai - 34 19. Al-Saudi Clinical Services (Citi Hospital) Adyar, Chennai - 20 20. CSI Kalyani General Hospital Dr. R.K.Salai, Chennai - 4 21. CSI Rainy Multi Speciality Hospital 45, GA Road, Chennai - 21 22. Deepam Hospitals (P) Ltd West Tambaram, Chennai - 45 23. Chennai Meenakshi Multispeciality Hospital Mylapore, Chennai - 4 24. Chennai National Hospital Beach Lane, Chennai - 1 25. Dr. Agarwal Eye Hospital Cathedral Road, Chennai - 86 26. Dr. A. -

Medical Research Foundation Notice
MEDICAL RESEARCH FOUNDATION To The Members of the Medical Research Foundation 7th September 2011 NOTICE NOTICE IS HEREBY GIVEN TO THE MEMBERS OF THE MEDICAL RESEARCH FOUNDATION THAT THE 33RD ANNUAL GENERAL MEETING OF THE FOUNDATION WILL BE HELD ON THRUSDAY, THE 29th SEPTEMBER 2011 AT 6.00 P.M. AT BOARD ROOM, 2ND FLOOR, KAMALNAYAN BAJAJ RESEARCH CENTRE BLOCK, NEW NO 41, OLD NO 18, COLLEGE ROAD, CHENNAI 600 006. THE AGENDA FOR THE MEETING IS GIVEN BELOW: AGENDA 1. To consider and adopt the Annual report of the Foundation for the year 2010-11. 2. To consider and adopt the Audited Income and Expenditure Account for the year ended March 31, 2011 and the Balance Sheet as at that date together with the Report of the Auditors thereon. 3. To appoint Auditors and to fix their remuneration for the year ending 31st March 2012. 4. To elect Members to the Board of Management in the vacancies caused by the retirement of the following Members, who are eligible for re-appointment. Name Category Mr. H D Malesra Patron Member Mr.Mani S Subramonian Patron Member 5. To elect Members to the Board of Management in the existing vacancy. Kindly make it convenient to attend the meeting. N.SUGALCHAND JAIN HONY. SECRETARY & TREASURER 1 MEDICAL RESEARCH FOUNDATION SANKARA NETHRALAYA MISSION STATEMENT QUALITY OBJECTIVES The mission of Sankara Nethralaya is to To maintain the quality of ophthalmic provide Total Eye-care solutions of highest s e r v i c e s i n a c c o r d a n c e w i t h standards to all sections of community international standards. -

2019 UICC Fellowship Programme Chairs and Review Committee Members
2019 UICC Fellowship Programme Chairs and Review Committee members The UICC has recruited a community of over 800 expert international reviewers who generously contribute their expertise to ensure the high-level peer review of applications whilst taking into account the international cross-cultural nature of the UICC Fellowship programmes. UICC would like to warmly thank the UICC fellowship Programme Chairs for their committed support and leadership on which our fellowship programmes depend. Fellowship Programme Chairs Professor W. Nicol Keith Professor of Molecular Oncology (Experimental Therapeutics) at the Institute of Cancer Sciences, Glasgow University, United Kingdom. Group Leader: Telomerase Therapeutics Group Professor Keith chairs the Yamagiwa-Yoshida Memorial International study grants review committee and co- chairs the Technical Fellowship Programme. Professor Robert Jones Professor of Clinical Cancer Research at the Institute of Cancer Sciences, Glasgow University, United Kingdom. Medical Oncologist at the University of Glasgow, Senior Lecturer in Clinical Pharmacology, Honorary Consultant Cancer Research UK, Beatson Laboratories. Professor Jones serves as Co-Chair of the Technical Fellowship Programme. Professor Abdellatif Benider Head doctor at the Centre Mohammed VI pour le traitement des cancers au Centre Hospitalier Universitaire Ibn Rochd Casablanca, Morocco. Professor Benider chairs the Bourses pour l’Afrique Francophone programme (BAF). UICC would also like to thank the following international review committee members -
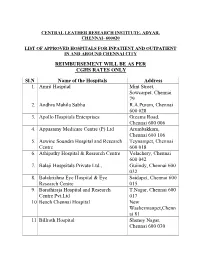
REIMBURSEMENT WILL BE AS PER CGHS RATES ONLY Sl.N Name Of
CENTRAL LEATHER RESEARCH INSTITUTE, ADYAR, CHENNAI- 600020 LIST OF APPROVED HOSPITALS FOR INPATIENT AND OUTPATIENT IN AND AROUND CHENNAI CITY REIMBURSEMENT WILL BE AS PER CGHS RATES ONLY Sl.N Name of the Hospitals Address 1. Amrit Hospital Mint Street, Sowcarpet, Chennai 79 2. Andhra Mahila Sabha R.A.Puram, Chennai 600 028 3. Apollo Hospitals Enterprises Greams Road, Chennai 600 006 4. Appasamy Medicare Centre (P) Ltd Arumbakkam, Chennai 600 106 5. Aswine Soundra Hospital and Research Teynampet, Chennai Centre 600 018 6. Athipathy Hospital & Research Centre Velachery, Chennai 600 042 7. Balaji Hospsitals Private Ltd., Guiindy, Chennai 600 032 8. Balakrishna Eye Hospital & Eye Saidapet, Chennai 600 Research Centre 015 9. Barathiraja Hospital and Research T.Nagar, Chennai 600 Centre Pvt.Ltd 017 10. Beach Chennai Hospital New Washermanpet,Chenn ai 81 11. Billroth Hospital Shenoy Nagar, Chennai 600 030 12. BSS Hospital Mandaveli, Chennai 600 028 13. Cancer Institute (WIA) Adyar, Chennai 600 020 14. Chettinad Health City Kelambakkam, Chennai 15. Child Trust Hospital Nungambakkam, 600 034 16. City Hospital Adyar, Chennai 600 020 17. CSI Kalyani General Hospital Dr. R.K.Salai, Chennai 600 004 18. CSI Rainy Multi Speciality Hospital 45 GA Road, Chennai 600 021 19. Deepam Hospitals (P) Ltd., West Tambaram, Chennai 600 045 20. Devaki Hospital Ltd Mylapore, Chennai 600 004 21. Dr. Agarwa l Eye Hospital Cathedral Road, Chennai 600 086 22. Dr. Meenakshi Hospitals Avadi, Chennai 600 054 23. Dr. Rabindran’s Health Care Centre Pvt. Ambattur, Chennai Ltd., 600 053 24. Dr. Rai Memorial Medical Centre Anna Salai, Chennai 600 018 25. -

Cancer Control
www.swaniti.in Cancer Control According to WHO, every year 1 million Indians are diagnosed with cancer. Currently the doctor –patient ratio for cancer treatment is at 1:2000. The Health Ministry aims to take it to 1:1000 by 2021. 27 Regional Cancer Centres (RCCs) providing comprehensive cancer care services had been set up under the National Cancer Control Programme (List of RCCs attached in the Annexure). However, with the goal mentioned above there is an urgent need to further increase the number of Cancer Centres in India. The 12th Plan has identified the need to strengthen tertiary care under National Program for Prevention and Control of Cancer, Diabetes, CVD and Stroke. Ministry of Health and Family Welfare will implement the scheme. Under the scheme, 20 State Cancer Institutes (SCI) in 20 States and 50 Tertiary Cancer Care Centres across the country will be established. The broad aim is to provide universal access for comprehensive cancer care. Framework for Implementation The Scheme will provide assistance to existing medical colleges and institutions. Autonomous institutions under Central or State Government will be eligible. NGOs with experience and expertise in tertiary care for cancer are also eligible. State/ UT Governments would recommend the proposals which are fulfilling the eligibility criteria, along with furnishing the commitment to provide State share of funds. The Institution will submit the proposal through State Governments including action plan for procurement of equipment, instruments related to cancer treatment and research. Proposal would be appraised by the Ministry of Health and Family Welfare. A tripartite Memorandum of Understanding (MOU) will be signed by the Institution, the State Government and Central Government before release of financial assistance. -

National Cancer Control Programme Guidelines
NATIONAL CANCER CONTROL PROGRAMME GUIDELINES MINISTRY OF HEALTH AND FAMILY WELFARE GOVERNMENT OF INDIA MAY 2005 Preface 1 Page No. Guidelines for New Regional The National Cancer Control Programme Cancer Centres 1 was initiated in the year 1975. Subsequently it was revised in the year 1984-85 with emphasis 2 on primary prevention and early detection of can- cer. Various schemes were introduced under the Guidelines for Existing Regional Cancer Centres 15 programme in order to strengthen cancer control activities in the country. Under the Xth Five Year Plan, the schemes have been modified to further 3 augment the exisiting facilities. The focus is on Guidelines for Development covering the geographical gaps for providing com- of Oncology Wing 28 prehensive cancer care in the country. 4 This document is a compendium of the re- Guidelines for District Cancer vised guidelines for the schemes under the NCCP. Control Programme 40 5 Guidelines for Decentralised NGO Scheme 54 1 Guidelines for New Regional Cancer Centres Introduction The National Cancer Control Programme has established Regional Cancer Centres (RCCs) to improve availability of cancer treatment fa- cilities. In order to further enhance the treatment facilities across the country and reduce the geographical gap in the availability of cancer care facilities, newer RCCs are being recognized. Provisions under the scheme 1) A one time financial assistance of upto Rs.Five crores would be provided to the new RCCs for development of cancer treatment facilities. This may include: a) Purchase of equipment related to cancer treatment and research b) Construction for housing the equipment or expanding the cancer wards 2) A part of the grant, not exceeding 30% of the total grant may be used if required, for construction of building to house the radio- therapy equipments and patient care units. -

(WIA) COLLEGE of ONCOLOGICAL SCIENCES Adyar, Chennai 600
CANCER INSTITUTE (WIA) Signed photograph to COLLEGE OF ONCOLOGICAL SCIENCES be pasted Adyar, Chennai 600 020 APPLICATION FOR ADMISSION TO MD (RADIOTHERAPY) / DMRT / BOTH MD & DMRT COURSE(S) - (2017-18 SESSION) 1 Name in BLOCK LETTERS with expanded initials at the end. Women candidates should add Selvi / Tmt. as the case may be. Name of the candidate should be underlined. 2 a) Complete address to which any communication is to be sent. b) Telephone no. (with code), if any c) Mobile No., if any d) E-mail id, if any. e) Fax no. (with code), if any. 3 Have you applied for any other course? If so, give details & order of priority. 4 Have you applied for any job? If so, give details & order of priority. 5 Nationality. (Evidence to be produced) 6 a) Present occupation, if any. b) Official address. c) Are you a deputed candidate? (If so, the necessary communication should be enclosed. If not a deputed candidate, a ‘No Objection Certificate’ from the employer should be enclosed) 7 Name, occupation & address of father / mother / husband / wife. 8 Do you belong to the scheduled caste / schedule tribe / socially & educationally backward classes specified in the Tamil Nadu Education Rules? (Evidence to be produced) 9 Date of birth & Age. (Evidence to be produced) 10 Mother tongue. 11 Medical qualification(s). 12 MBBS degree: a) College from which qualified. b) University from which qualified. c) Whether the University is recognized by Medical Council of India / the Tamil Nadu Dr MGR Medical University. d) Register number of the qualifying examination. e) Month & year of passing.