Bioorganic Chemistry 82 (2019) Ii-Xiv
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Governing Board Meeting 1-2 April 2015 – Association of Arab Universities
Euro-Mediterranean Universities Network TETHYS Governing Board Meeting 1-2 april 2015 – Association of Arab Universities A ce jour (6 mars 2015), le Consortium Téthys regroupe 76 universités réparties dans 17 pays du pourtour méditerranéen ALGERIE JORDANIE The Tethys Network Université Benyoucef Benkhedda - Alger Université Philadelphia - Amman Université Abderrahmane Mira - Béjaïa Université de Technologie Princesse Sumaya - Amman Université d’Oran Université de Mutah Université Badji Mokhtar - Annaba Université de Yarmouk Université du 08 Mai 1945 - Guelma Université Jordanienne de Science et technologie - Irbid Université du 20 Août 1955 - Skikda Université de Jordanie – Amman Université Larbi Ben M’hidi - Oum El Bouaghi Université Mohamed Khider - Biskra Université Constantine I LIBAN Université Constantine II Université Constantine III Université Saint-Esprit de Kaslik-Jounieh Today, the Tethys Network is Université d’Alger 2 Université Saint Joseph - Beyrouth Université de Balamand - Tripoli Université Libanaise – Beyrouth composed of 76 universities from 17 CHYPRE Université de Chypre - Nicosie LIBYE countries of the Mediterranean Université de Zawia CROATIE Université de Split Basin Université de Zagreb MALTE Université de Malte EGYPTE Université d’Alexandrie Université d’Assiut MAROC Université d’Helwan Université Abdelmalek Essaâdi - Tanger Université du Caire Université Chouaïb Doukkali - El Jadida MUST Université Science et Technologie - Le Caire Université Cadi Ayyad - Marrakech Université Française d’Egypte Université Euro-Méditerranéenne -

Report on the International Workshop on the Complex Turbulent Flows Tangier-Morocco, November 27-28, 2017
Report on the International Workshop on the Complex Turbulent Flows Tangier-Morocco, November 27-28, 2017 Otman Ben Mahjoub and Aziz Ouadoud Department of Physics Polydisciplinary Faculty of Larache Abdelmalek Essaadi University 92004 Larache, Morocco 1 Introduction 2 Participants The workshop attracted a total of 86 scientists from In the framework of scientific events, the Polydisci- 25 countries, including 35 PhD students as shown plinary Faculty of Larache and the University Abdel- in table1. Most participants came from European malek Essaadi in collaboration with the ERCOFTAC universities or research institutes. organized the international Workshop on the Com- plex Turbulent Flows held in Tangier, Morocco from ALGERIE 03 BELGUIM 02 the 27th to 28th of November 2017. The object of the BRESIL 01 CANADA 02 workshop was to deal with issues of turbulent and CHILE 01 DENMARK 01 complex flows, especially with the planet’s sudden EGYPT 01 FRANCE 09 climate change in oceans and atmosphere, which GERMANY 02 INDIA 01 have highly negative impacts on the sustainable ITALY 03 MOROCCO 26 development and management of resources. The NETHERLAND 02 POLAND 01 workshop brought together experimentalists, nu- REPUBLIC 01 RUSSIA 03 mericists and theoreticians from around the world CZECH with experts in turbulent flow simulations, compu- SINGAPORE 01 SPAIN 09 tational mathematics, and high-performance com- SWEDEN 01 SWISS 01 puting. Participants presented and discussed recent TUNISIE 08 UK 05 advances in the field of turbulence and topics re- USA 02 lated to the study of chaos, nonlinear dynamical systems of turbulent flows, and their application to atmospheric and oceanic flows. -

World Higher Education Database Whed Iau Unesco
WORLD HIGHER EDUCATION DATABASE WHED IAU UNESCO Página 1 de 438 WORLD HIGHER EDUCATION DATABASE WHED IAU UNESCO Education Worldwide // Published by UNESCO "UNION NACIONAL DE EDUCACION SUPERIOR CONTINUA ORGANIZADA" "NATIONAL UNION OF CONTINUOUS ORGANIZED HIGHER EDUCATION" IAU International Alliance of Universities // International Handbook of Universities © UNESCO UNION NACIONAL DE EDUCACION SUPERIOR CONTINUA ORGANIZADA 2017 www.unesco.vg No paragraph of this publication may be reproduced, copied or transmitted without written permission. While every care has been taken in compiling the information contained in this publication, neither the publishers nor the editor can accept any responsibility for any errors or omissions therein. Edited by the UNESCO Information Centre on Higher Education, International Alliance of Universities Division [email protected] Director: Prof. Daniel Odin (Ph.D.) Manager, Reference Publications: Jeremié Anotoine 90 Main Street, P.O. Box 3099 Road Town, Tortola // British Virgin Islands Published 2017 by UNESCO CENTRE and Companies and representatives throughout the world. Contains the names of all Universities and University level institutions, as provided to IAU (International Alliance of Universities Division [email protected] ) by National authorities and competent bodies from 196 countries around the world. The list contains over 18.000 University level institutions from 196 countries and territories. Página 2 de 438 WORLD HIGHER EDUCATION DATABASE WHED IAU UNESCO World Higher Education Database Division [email protected] -

Registered Attendees
Registered Attendees Company Name Job Title Country/Region 1996 Graduate Trainee (Aquaculturist) Zambia 1Life MI Manager South Africa 27four Executive South Africa Sales & Marketing: Microsoft 28twelve consulting Technologies United States 2degrees ETL Developer New Zealand SaaS (Software as a Service) 2U Adminstrator South Africa 4 POINT ZERO INVEST HOLDINGS PROJECT MANAGER South Africa 4GIS Chief Data Scientist South Africa Lead - Product Development - Data 4Sight Enablement, BI & Analytics South Africa 4Teck IT Software Developer Botswana 4Teck IT (PTY) LTD Information Technology Consultant Botswana 4TeckIT (pty) Ltd Director of Operations Botswana 8110195216089 System and Data South Africa Analyst Customer Value 9Mobile Management & BI Nigeria Analyst, Customer Value 9mobile Management Nigeria 9mobile Nigeria (formerly Etisalat Specialist, Product Research & Nigeria). Marketing. Nigeria Head of marketing and A and A utilities limited communications Nigeria A3 Remote Monitoring Technologies Research Intern India AAA Consult Analyst Nigeria Aaitt Holdings pvt ltd Business Administrator South Africa Aarix (Pty) Ltd Managing Director South Africa AB Microfinance Bank Business Data Analyst Nigeria ABA DBA Egypt Abc Data Analyst Vietnam ABEO International SAP Consultant Vietnam Ab-inbev Senior Data Analyst South Africa Solution Architect & CTO (Data & ABLNY Technologies AI Products) Turkey Senior Development Engineer - Big ABN AMRO Bank N.V. Data South Africa ABna Conseils Data/Analytics Lead Architect Canada ABS Senior SAP Business One -

Enhanced Antibacterial Efficiency of Cellulosic Fibers: Microencapsulation and Green Grafting Strategies
coatings Article Enhanced Antibacterial Efficiency of Cellulosic Fibers: Microencapsulation and Green Grafting Strategies Dorra Dridi 1,†, Aicha Bouaziz 2,3,†, Sondes Gargoubi 4,*, Abir Zouari 5, Fatma B’chir 6, Aghleb Bartegi 2, Hatem Majdoub 7 and Chedly Boudokhane 5 1 Unit of Analysis and Process Applied to the Environment (UR17ES32) Issat Mahdia, Department of Environmental Sciences & Nutrition, University of Monastir, Monastir 5000, Tunisia; [email protected] 2 Bio-Resources, Integrative Biology & Valorization (BIOLIVAL, LR14ES06), Higher Institute of Biotechnology of Monastir, University of Monastir, Monastir 5000, Tunisia; [email protected] (A.B.); [email protected] (A.B.) 3 Higher School of Health Sciences and Techniques of Sousse, University of Sousse, Sousse 4054, Tunisia 4 Textile Engineering Laboratory—LGTex, Textile Department, University of Monastir, Monastir 5000, Tunisia 5 Research Unity of Applied Chemistry and Environment, Faculty of Science of Monastir, University of Monastir, Monastir 5000, Tunisia; [email protected] (A.Z.); [email protected] (C.B.) 6 Research Department, Institute National de Recherche et d’Analyse Physico-Chimique-Pôle Technologique de Sidi Thabet, Sidi Thabet 2020, Tunisia; [email protected] 7 Laboratory of Advanced Materials and Interfaces, Faculty of Sciences of Monastir, University of Monastir, Monastir 5000, Tunisia; [email protected] * Correspondence: [email protected] † D. Dridi and A. Bouaziz contributed equally to this work as first authors. Abstract: We report an analysis of chemical components of essential oils from barks of Ceylon cinnamon and cloves of Syzygium aromaticum and an investigation of their antibacterial activity. The components Citation: Dridi, D.; Bouaziz, A.; of oils were determined by using Gas Chromatography/Mass Spectrometry (GC-MS) analysis, and Gargoubi, S.; Zouari, A.; B’chir, F.; the antimicrobial activity was assessed by the disk diffusion test. -

Adaptation of AL's Model to Foreign Countries
Programme HERE - Technical Assistance Mission “European experience of employment of graduates: Student support services for employability” Ferghana State University 10-11 July 2018 « The international dimension of AlmaLaurea: adaptation of the Italian model to foreign countries in the framework of Erasmus+ CBHE projects » Enrico Dongiovanni Project Manager for International Relations AlmaLaurea Interuniversity Consortium Outline The Erasmus+ CBHE TUNED Project: TUnisian Network for Employability and Development of graduates skills General overview Objectives Project partnership Working packages Deliverables & Indicators Mobility schedule General overview • Programme: Erasmus+, KA2 – CBHE Structural project • Duration: 36 months Start of the eligibility period: 15/10/2016 End of the eligibility period: 14/10/2019 • Partnership: 8 Tunisian Universities; Tunisian Ministry of Higher Education; 5 EU partners; 4 associated partners • Beneficiary country: Tunisia • Grant awarded: 688.010 € Objectives • Wider objective: To improve the match between universities education and the labour market requirements in Tunisia, enhancing the efficiency of the whole HE system by a certified system of QA and the monitoring of HE programmes and graduates • Specific objectives: To build Tunisian capacities by transferring EU best practices on graduates' employability and monitoring of universities' performances To enhance the empowerment of Tunisian universities To strengthen university/labour market linkages To increase the collaboration among Universities -
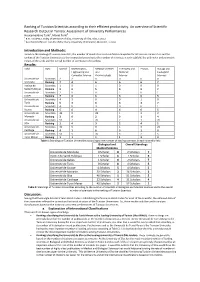
Ranking of Tunisian Scientists According to Their Efficient Productivity
Ranking of Tunisian Scientists according to their efficient productivity. An overview of Scientific Research Output in Tunisia: Assessment of University Performances Houcemeddine Turkia, Manel Turkib a B.Sc. Student, Faculty of Medicine of Sfax, University of Sfax, Sfax, Tunisia b Assistant Professor. Faculty of Pharmacy, University of Monastir, Monastir, Tunisia Introduction and Methods: Based on the Ranking of Tunisian Scientists, the number of scientists in each subfield is computed for all Tunisian Universities and the ranking of the Tunisian Universities is then computed according to the number of scientists in each subfield, the arithmetic and geometric means of the ranks and the overall number of scientists in the ranking. Results: Field Data Overall Mathematics, Medical Sciences Chemistry and Physics Biology and Engineering and and Material Geological Computer Science Pharmacology Science Sciences Université de Scientists 2 2 0 0 0 0 Manouba Ranking 7 4 6 6 6 7 Institut de Scientists 1 0 1 0 0 0 Santé Publique Ranking 9 6 5 6 6 7 Université de Scientists 2 0 0 1 0 1 Gabès Ranking 7 6 6 3 6 5 Université de Scientists 4 3 0 0 1 0 Tunis Ranking 5 3 6 6 4 7 Université de Scientists 4 0 3 0 0 1 Sousse Ranking 5 6 4 6 6 5 Université de Scientists 34 0 28 1 3 2 Monastir Ranking 3 6 2 3 1 4 Université de Scientists 51 2 20 2 1 26 Sfax Ranking 2 4 3 2 4 1 Université de Scientists 31 5 0 1 3 22 Carthage Ranking 4 1 6 3 1 2 Université de Scientists 52 5 35 5 2 5 Tunis Manar Ranking 1 1 1 1 3 3 Table 1: Standings of Tunisian Universities according -

Minufiya Veterinary Journal ISSN: 1110-9750
Minufiya Veterinary Journal ISSN: 1110-9750 Volume 9 September 2015 ISSN (prINt): 1110-9750 published by Faculty oF Veterinary medIcine University oF Sadat City egypt Email: [email protected] Minufiya Veterinary Journal ISSN: 1110-9750 Vol. 9; September 2015 ﻣﺠﻠﺲ إدارة اﻟﻤﺠﻠﺔ رﺋﯿﺲ ﻣﺠﻠﺲ إدارة اﻟﻤﺠﻠﺔ م اﻹﺳﻢ اﻟﻮظﯿﻔﺔ اﻟﺼﻔﺔ ۱ ا.د/ ﺷﻌﺒﺎن ﻣﺤﻤﺪ ﺟﺎد ﷲ ﻋﻤﯿﺪ اﻟﻜﻠﯿﺔ رﺋﯿﺲ ﻣﺠﻠﺲ اﻹدارة ھﯿﺌﺔ اﻟﺘﺤﺮﯾﺮ م اﻹﺳﻢ اﻟﻮظﯿﻔﺔ اﻟﺼﻔﺔ ۱ أ.د/ أﺣﻤﺪ ﻋﺒﺪ اﻟﻤﻨﻌﻢ زﻏﺎوة أﺳﺘﺎذ اﻷﻣﺮاض اﻟﺒﺎطﻨﺔ واﻟﻤﻌﺪﯾﺔ رﺋﯿﺲ ﺗﺤﺮﯾﺮ اﻟﻤﺠﻠﺔ ۲ أ.د/ ﺳﻌﺪ ﻋﺒﺪ اﻟﻔﺘﺎح ﻋﻤﺎره أﺳﺘﺎذ اﻟﺨﻠﯿﺔ و اﻷﻧﺴﺠﺔ ﻋﻀﻮا ً ۳ ا.د/ ﻣﺤﻤﺪ ﻋﺎطﻒ ھﻼل أﺳﺘﺎذ اﻟﺮﻋﺎﯾﺔ وﺗﻨﻤﯿﺔ اﻟﺜﺮوة اﻟﺤﯿﻮاﻧﯿﺔ ﻋﻀﻮا ً ٤ ا.د/ ﺣﻤﺎده ﺿﺎﺣﻲ ﺣﺴﯿﻦ أﺳﺘﺎذ اﻟﺮﻋﺎﯾﺔ وﺗﻨﻤﯿﺔ اﻟﺜﺮوة اﻟﺤﯿﻮاﻧﯿﺔ ﻋﻀﻮا ً ٥ أ.د/ ﺧﺎﻟﺪ ﻣﺤﻤﻮد ﻣﺤﻤﺪ ﺟﻌﻔﺮ أﺳﺘﺎذ اﻟﺘﻐﺬﯾﺔ واﻟﺘﻐﺬﯾﺔ اﻹﻛﻠﯿﻨﯿﻜﯿﺔ ﻋﻀﻮا ً ٦ د/ ﻋﻤﺎد ﻣﺤﻤﻮد ﻣﺤﻤﺪ ﻋﺒﺪ اﻟﺮازق أﺳﺘﺎذ ﻣﺴﺎﻋﺪ اﻟﺘﻮﻟﯿﺪ واﻟﺘﻨﺎﺳﻞ واﻟﺘﻠﻘﯿﺢ اﻻﺻﻄﻨﺎﻋﻲ ﻋﻀﻮا ً ۷ د. ﺷﺮﯾﻒ ﻋﺒﺪ ﷲ زﯾﺪان أﺳﺘﺎذ ﻣﺴﺎﻋﺪ ﺑﻘﺴﻢ اﻟﺼﺤﺔ واﻷﻣﺮاض اﻟﻤﺸﺘﺮﻛﺔ ﻋﻀﻮا ً ۸ د/ ﻣﺒﺮوك ﻋﻄﯿﺔ ﻋﺒﺪ اﻟﺪاﯾﻢ ﻣﺪرس اﻟﻜﯿﻤﯿﺎء اﻟﺤﯿﻮﯾﺔ وﻛﯿﻤﯿﺎء اﻟﺘﻐﺬﯾﺔ ﻋﻀﻮا ً ۹ د/ ﻣﺤﻤﺪ أﺑﻮ اﻟﻌﺰ ﻧﺎﯾﻞ ﻣﺪرس اﻷﻣﺮاض اﻟﺒﺎطﻨﺔ واﻟﻤﻌﺪﯾﺔ ﻋﻀﻮا ً ۱۰ د/ أﺣﻤﺪ ﻣﺤﻤﻮد ﻋﺒﺪ اﻟﻮاﺣﺪ اﻟﺼﯿﻔﻲ ﻣﺪرس اﻷﻣﺮاض اﻟﺒﺎطﻨﺔ واﻟﻤﻌﺪﯾﺔ ﻋﻀﻮا ً ۱۱ د/ أﻛﺮم أﺣﻤﺪ ﺣﺴﻨﯿﻦ ﺳﻼﻣﺔ ﻣﺪرس اﻷﻣﺮاض اﻟﺒﺎطﻨﺔ واﻟﻤﻌﺪﯾﺔ ﻋﻀﻮا ً ۱۲ د/ ﻣﺼﻄﻔﻰ ﻋﺒﺪ اﻟﺠﺎﺑﺮ ﻣﺪرس اﻟﺒﺎﺛﻮﻟﻮﺟﯿﺎ ﻋﻀﻮا ً ۱۳ د/ رﺑﯿﻊ اﻟﺤﺴﯿﻨﻲ اﻣﺒﺎرك ﻣﺪرس اﻟﺮﻗﺎﺑﺔ اﻟﺼﺤﯿﺔ ﻋﻠﻰ اﻷﻏﺬﯾﺔ ﻋﻀﻮا ً ۱٤ ط.ب / ﻋﺒﺎس ﻓﺘﺤﻲ ﻋﺒﺎس ﻣﺼﺮي ﻣﻌﯿﺪ اﻟﺠﺮاﺣﺔ واﻟﺘﺨﺪﯾﺮ واﻷﺷﻌﺔ ﻋﻀﻮا ً ۱٥ ط.ب/ ﻋﺒﺪ اﻟﻔﺘﺎح ﻣﺤﻤﺪ ﻋﺒﺪ اﻟﻔﺘﺎح ﻣﻌﯿﺪ اﻟﺒﺎﺛﻮﻟﻮﺟﯿﺎ اﻹﻛﻠﯿﻨﯿﻜﯿﺔ ﻋﻀﻮا ً ۱٦ أ. -

The Internationalisation of Higher Education in the Mediterranean CURRENT and PROSPECTIVE TRENDS
The Internationalisation of Higher Education in the Mediterranean CURRENT AND PROSPECTIVE TRENDS @2021 Union for the Mediterranean Address: Union for the Mediterranean [UfM] ufmsecretariat Palacio de Pedralbes @UfMSecretariat Pere Duran Farell, 11 ES-08034 Barcelona, Spain union-for-the-mediterranean Web: http://www.ufmsecretariat.org @ufmsecretariat Higher Education & Research Phone: +34 93 521 41 51 E-mail: [email protected] Authors: (in alphabetical order): Maria Giulia Ballatore, Raniero Chelli, Federica De Giorgi, Marco Di Donato, Federica Li Muli, Silvia Marchionne, Anne-Laurence Pastorini, Eugenio Platania, Martina Zipoli Coordination: Marco Di Donato, UNIMED; João Lobo, UfM Advisory: Itaf Ben Abdallah, UfM Creative layout: kapusons Download publication: https://ufmsecretariat.org/info-center/publications/ How to cite this publication: UNIMED (2021). The Internationalisation of Higher Education in the Mediterranean, Current and prospective trends. Barcelona: Union for the Mediterranean Disclaimer: Neither the Union for the Mediterranean nor any person acting on behalf of the Union for the Mediterranean is responsible for the use that might be made of the information contained in this report. The information and views set out in this report do not reflect the official opinion of the Union for the Mediterranean. Responsibility for the information and views expressed therein lies entirely with the authors. All care has been taken by the authors to ensure that, where necessary, permission was obtained to use any parts of manuscripts including illustrations, maps and graphs on which intellectual property rights already exist from the titular holder(s) of such rights or from her/his or their legal representative. Copyright: © Union for the Mediterranean, 2021 Reproduction is authorised provided the source is acknowledged. -

The Integration of Multivariate Statistical Approaches, Hyperspectral Reflectance, and Data-Driven Modeling for Assessing the Qu
water Article The Integration of Multivariate Statistical Approaches, Hyperspectral Reflectance, and Data-Driven Modeling for Assessing the Quality and Suitability of Groundwater for Irrigation Mosaad Khadr 1,2 , Mohamed Gad 3 , Salah El-Hendawy 4,5,*, Nasser Al-Suhaibani 4, Yaser Hassan Dewir 4,6 , Muhammad Usman Tahir 4, Muhammad Mubushar 4 and Salah Elsayed 7 1 Civil Engineering Department, College of Engineering, University of Bisha, Bisha 61922, Saudi Arabia; [email protected] 2 Irrigation and Hydraulics Department, Faculty of Engineering, Tanta University, Tanta 31734, Egypt 3 Hydrogeology, Evaluation of Natural Resources Department, Environmental Studies and Research Institute (ESRI), University of Sadat City, Minufiya 32897, Egypt; [email protected] 4 Department of Plant Production, College of Food and Agriculture Sciences, King Saud University, KSA, P.O. Box 2460, Riyadh 11451, Saudi Arabia; [email protected] (N.A.-S.); [email protected] (Y.H.D.); [email protected] (M.U.T.); [email protected] (M.M.) 5 Department of Agronomy, Faculty of Agriculture, Suez Canal University, Ismailia 41522, Egypt 6 Faculty of Agriculture, Kafrelsheikh University, Kafr El-Sheikh 33516, Egypt 7 Agricultural Engineering, Evaluation of Natural Resources Department, Environmental Studies and Research Institute, University of Sadat City, Minufiya 32897, Egypt; [email protected] * Correspondence: [email protected]; Tel.: +966-535318364 Abstract: Sustainable agriculture in arid regions necessitates that the quality of groundwater be care- Citation: Khadr, M.; Gad, M.; fully monitored; otherwise, low-quality irrigation water may cause soil degradation and negatively El-Hendawy, S.; Al-Suhaibani, N.; impact crop productivity. -

Virulence Determinants and Antimicrobial Profiles of Pasteurella Multocida Isolated from Cattle and Humans in Egypt
antibiotics Article Virulence Determinants and Antimicrobial Profiles of Pasteurella multocida Isolated from Cattle and Humans in Egypt Mohamed Sabry Abd Elraheam Elsayed 1,*, Samah Mahmoud Eldsouky 2, Tamer Roshdy 3, Lamia Said 4, Nahed Thabet 4, Tamer Allam 4, A. B. Abeer Mohammed 5, Ghada M. Nasr 6, Mohamed S. M. Basiouny 7, Behairy A. Akl 8, Maha M. Nader 8, Al Shaimaa Hasan 9 and Ahmed Salah 3 1 Department of Bacteriology, Mycology, and Immunology, Faculty of Veterinary Medicine, University of Sadat City, Sadat City, Minufiya 32897, Egypt 2 Department of Otolaryngology and Head and Neck Surgery, Faculty of Medicine, Benha University, Benha City, Qalyubia 13511, Egypt; [email protected] 3 Department of Molecular Biology, Genetic Engineering and Biotechnology Research Institute, University of Sadat City, Sadat City, Minufiya 32897, Egypt; [email protected] (T.R.); [email protected] (A.S.) 4 Department of Clinical Pathology, Faculty of Veterinary Medicine, University of Sadat City, Sadat City, Minufiya 32897, Egypt; [email protected] (L.S.); [email protected] (N.T.); [email protected] (T.A.) 5 Department of Microbial Biotechnology, Genetic Engineering and Biotechnology Research Institute, University of Sadat City, Sadat City, Minufiya 32897, Egypt; [email protected] 6 Department of Molecular Diagnostics, Genetic Engineering and Biotechnology Research Institute, Citation: Elsayed, M.S.A.E.; University of Sadat City, Sadat City, Minufiya 32897, Egypt; [email protected] Eldsouky, S.M.; Roshdy, T.; Said, L.; 7 Faculty of Biotechnology, Badr University, Badr City, Cairo 19592, Egypt; [email protected] Thabet, N.; Allam, T.; Mohammed, 8 Microbiology Department, Faculty of Agriculture, Zagazig University, Zagazig 44519, Egypt; A.B.A.; Nasr, G.M.; Basiouny, M.S.M.; [email protected] (B.A.A.); [email protected] (M.M.N.) Akl, B.A.; et al. -

Bibliometric Study of African Publications in Dental Medicine in Indexed Journals Between 2008 and 2018
Research Article ISSN 2639-9490 Research Article Oral Health & Dental Science Bibliometric Study of African Publications in Dental Medicine in Indexed Journals between 2008 and 2018 Bennani A1*, Hamza M2, Kalali C3 and Jari A4 1Professor of Department of fixed prosthodontics, Faculty of Dentistry, University Hassan II, Casablanca, Morocco. *Correspondence: 2Professor of Department of Epidemiology and biostatistics, Bennani A, Professor of Department of fixed prosthodontics, Faculty of Dentistry, University Hassan II, Casablanca, Morocco. Faculty of Dentistry, University Hassan II, Casablanca, Morocco. 3Dentist, Private practice, Casablanca, Morocco. Received: 20 December 2020; Accepted: 07 January 2021 4Dentist, Private practice, Casablanca, Morocco. Citation: Bennani A, Hamza M, Kalali C, et al. Bibliometric Study of African Publications in Dental Medicine in Indexed Journals between 2008 and 2018. Oral Health Dental Sci. 2021; 4(3); 1-12. ABSTRACT Introduction: The objective of this work is to make a bibliometric analysis of publications in odontology of African universities between January 2008 and December 2018 based on publications published in indexed journals on Pubmed. Material and Methods: Our work is a comprehensive retrospective descriptive study intended to carry out a bibliometric analysis of articles in odontology published between January 1, 2008 and December 31, 2018 by professor researchers from African universities. For each selected article, we determined the authors, the title, their affiliations, the year of publication. Results: Based on our sample of 19 African countries (Morocco, Algeria, Tunisia, Egypt, Nigeria, Cameroon, Côte d 'Ivoire, Ethiopia, Ghana, Guinea, Kenya, Libya, Madagascar, Mali, South Africa, DRC Congo, Senegal, Sudan, Zimbabwe) that included 66 faculties; the results of our study showed: • In terms of the number of global publications, Egypt, Nigeria, Morocco and South Africa are the leading countries.