Dinosaur Egg Parataxonomy [1]
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Oogenesis and Mode of Reproduction in the Soybean Cyst Nematode, Heterodera Glycines1)
OOGENESIS AND MODE OF REPRODUCTION IN THE SOYBEAN CYST NEMATODE, HETERODERA GLYCINES1) BY A. C. TRIANTAPHYLLOU and HEDWIG HIRSCHMANN Departments of Genetics and Plant Pathology, North Carolina State College, Raleigh, North Carolina, U.S.A. Oögenesis and mode of reproduction were studied in four populations of the soybean cyst nematode, Heterodera glycines. Oögonial divisions occurred before and during the fourth molt. Maturation of oöcytes proceeded only in inseminated females and was normal, consisting of two meiotic divisions and the formation of two polar nuclei. Nine bivalents were present at metaphase I in all populations. Sperm entered the oöcytes at late prophase or early metaphase I. Following the second maturation division, sperm and egg pronuclei fused to form the zygote nucleus. Six females obtained from 200 larval inoculations of soybean seedlings failed to produce embryonated eggs and showed marked retardation in growth. In conclusion, H. glycines has a normal meiotic cycle and reproduces by cross fertilization. "Prior to 1940, there was a strong tendency to refer all of the cyst-forming nematodes to a single species, Heterodera schachtii Schmidt ..." (Taylor, 1957 ) . Infraspecific categories identified on the basis of host preferences were later distinguished by slight morphological differences and were described as separate species. Although as many as fifteen or sixteen species have been recognized, the taxonomic situation is far from satisfactory. The specific rank of some species is questionable, whereas other species contain forms which may well deserve specific rank. Cytological and, furthermore, cytogenetical studies may elucidate the evolutionary relationships among the various Heterodera species. Information on various aspects of oogenesis of six Heterodera species is already available (Mulvey, 1957, 1958, 1960; Riley & Chapman, 1957; Cotten, 1960). -

Evaluating and Treating the Reproductive System
18_Reproductive.qxd 8/23/2005 11:44 AM Page 519 CHAPTER 18 Evaluating and Treating the Reproductive System HEATHER L. BOWLES, DVM, D ipl ABVP-A vian , Certified in Veterinary Acupuncture (C hi Institute ) Reproductive Embryology, Anatomy and Physiology FORMATION OF THE AVIAN GONADS AND REPRODUCTIVE ANATOMY The avian gonads arise from more than one embryonic source. The medulla or core arises from the meso- nephric ducts. The outer cortex arises from a thickening of peritoneum along the root of the dorsal mesentery within the primitive gonadal ridge. Mesodermal germ cells that arise from yolk-sac endoderm migrate into this gonadal ridge, forming the ovary. The cells are initially distributed equally to both sides. In the hen, these germ cells are then preferentially distributed to the left side, and migrate from the right to the left side as well.58 Some avian species do in fact have 2 ovaries, including the brown kiwi and several raptor species. Sexual differ- entiation begins by day 5 in passerines and domestic fowl and by day 11 in raptor species. Differentiation of the ovary is characterized by development of the cortex, while the medulla develops into the testis.30,58 As the embryo develops, the germ cells undergo three phases of oogenesis. During the first phase, the oogonia actively divide for a defined time period and then stop at the first prophase of the first maturation division. During the second phase, the germ cells grow in size to become primary oocytes. This occurs approximately at the time of hatch in domestic fowl. During the third phase, oocytes complete the first maturation division to 18_Reproductive.qxd 8/23/2005 11:44 AM Page 520 520 Clinical Avian Medicine - Volume II become secondary oocytes. -
![[PDF] Dinosaur Eggshell from the Red Sandstone Group of Tanzania](https://docslib.b-cdn.net/cover/9168/pdf-dinosaur-eggshell-from-the-red-sandstone-group-of-tanzania-179168.webp)
[PDF] Dinosaur Eggshell from the Red Sandstone Group of Tanzania
Journal of Vertebrate Paleontology 24(2):494±497, June 2004 q 2004 by the Society of Vertebrate Paleontology NOTE DINOSAUR EGGSHELL FROM THE RED SANDSTONE GROUP OF TANZANIA MICHAEL D. GOTTFRIED1, PATRICK M. O'CONNOR2, FRANKIE D. JACKSON3, ERIC M. ROBERTS4, and REMEGIUS CHAMI5, 1Mich- igan State University Museum, East Lansing, Michigan, 48824, [email protected]; 2College of Osteopathic Medicine, Ohio University, Athens, Ohio, 45701; 3Department of Earth Sciences, Montana State University, Bozeman, Montana, 59717; 4Department of Geology and Geophysics, University of Utah, Salt Lake City, Utah, 84112, 5Antiquities Unit, P.O. Box 2280, Dar es Salaam, Tanzania Investigations over the last several decades at Gondwanan Mesozoic Although the age of the Red Sandstone Group is poorly understood (see localities have signi®cantly expanded our knowledge of the diversity Damblon et al., 1998), a Cretaceous age is suggested at this site based and distribution of Southern Hemisphere dinosaurs. These records are on (1) the overall composition of the fauna, which includes titanosaurid? primarily based on skeletal remains, but included among them are in- sauropods and both avian and nonavian theropods, as well as osteo- stances of preserved eggshell, notably from Argentina (e.g., Calvo et glossomorph ®shes, and (2) the possibility that these deposits may be al., 1997; Chiappe et al., 1998) and India (e.g., Khosla and Sahni, 1995). approximately coeval with the Cretaceous dinosaur beds of Malawi (Ja- In general, however, dinosaur eggshell is relatively poorly known from cobs et al., 1990), which lie ca. 200 km southeast of the Mbeya region. Gondwana, and from Africa in particular. -

Sperm Storage in the Oviduct of the American Alligator DANIEL H
JOURNAL OF EXPERIMENTAL ZOOLOGY 309A:581–587 (2008) Sperm Storage in the Oviduct of the American Alligator DANIEL H. GIST1Ã, APRIL BAGWILL2, VALENTINE LANCE3, 2 4 DAVID M. SEVER , AND RUTH M. ELSEY 1Department of Biological Sciences, University of Cincinnati, Cincinnati, Ohio 2Department of Biological Sciences, Southeastern Louisiana University, Hammond, Louisiana 3San Diego State University, Graduate School of Public Health, San Diego, California 4Louisiana Department of Wildlife and Fisheries, Rockefeller Wildlife Refuge, Grand Chenier, Louisiana ABSTRACT Oviducts of the American alligator (Alligator mississippiensis) were examined histologically for the presence of stored sperm. Two regions containing sperm were identified, one at the junction of the posterior uterus and the vagina (UVJ) and the other at the junction of the tube and isthmus (TIJ). In these areas, sperm were found in the lumina of oviductal glands. The glands in these areas of the oviduct are diffuse and shallow and appear to allow better access to sperm than glands located elsewhere. Histochemically, the glands of the UVJ reacted weakly for carbohydrates and proteins, whereas those of the TIJ reacted strongly for these same two components, secretions of which are associated with sperm storage structures in other reptiles. Sperm were not in contact with the glandular epithelium, and glands at the UVJ contained more sperm than those at the TIJ. Oviductal sperm storage was observed not only in recently mated females but in all females possessing uterine eggs as well as all females known to be associated with a nest. We conclude that female alligators are capable of storing sperm in their oviductal glands, but not from one year to the next. -

Study on the Ovary, Oviduct And
T it le of the paper : STUDY ON THE OVARY, OVIDUCT AND UTERUS OF THE EWE. By: ROBERT HADEK, Dr.Med.Vet.-, Vienna. From: The Department of Veterinary Histology and Embryology of The University of Glasgow Veterinary School. Submitted as Thesis for the Degree of Bi.D. in the Faculty of Medicine. ProQuest Number: 13838881 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 13838881 Published by ProQuest LLC(2019). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 I A4 Contents V ol. I . Introduction Page 1 L iterature 1 M aterial & Methods Anatomical observations and measurements 5 H isto lo g ic a l and histochem ical technique 6 The breeding season and the sexual cy cle in the ewe 10 The ovary Gross Anatomy 11 H istology 12 Oogenesis and follicular development 14 The growth of the follicle and ovum 19 Multinuclear ova, polyovular follicles and accessory oocytes 20 Follicular degeneration and atresia 22 The rupture of the follicle 23 The corpus luteum 24 Histochemical reactions in the ovary 30 Histochemical reactions in the follicle -

Cairanoolithus: a Large Egg for a Small Dinosaur with Wide Hips
20/11/2020 <em>Cairanoolithus</em>: a Large Egg for a Small Dinosaur With Wide Hips - UABDivulga Barcelona Research & Innovation 02/2015 Cairanoolithus: a Large Egg for a Small Dinosaur With Wide Hips The study of the microstructure of the eggshell of Cairanoolithus conducted by Albert G. Sellés and Angel Galobart, both researchers at the Catalan Institute of Palaeontology Miquel Crusafont (ICP), reveals that this egg type does not belong to sauropod dinosaurs but to ankylosaurs, and probably to Struthiosaurus, a genus of armored dinosaurs. The finding would represent the first description of thyreophora eggs (a group that includes ankylosauria and stegosauria) in the world. It is an arduous task for paleontologists to assign the fossil eggs they found in excavations to a certain dinosaur species or group of dinosaurs, especially because the lack of bones of the parents in the nests and the low probability to find embryonic remains that would help in the taxonomic assignation of the eggs. Thus, we often do not known who laid a certain fossil egg. These limitations have led scientist to design a specific system for naming and classifying fossil eggs, using concepts such as "oogenera" and "ooespecies" for its phylogenetic classification. Cairanoolithus is an oogenus discovered and described in the early 90’s. It takes its name from the first place where it was found, a site near La Cairanne, a small town from southeastern France. From then on, Cairanoolithus has been found in 25 different sites. These eggs are between 72.2 and 71.4 million years old, they are large (over 15 cm in diameter) and have a rounded shape. -

ASC-201: Avian Female Reproductive System
COOPERATIVE EXTENSION SERVICE UNIVERSITY OF KENTUCKY COLLEGE OF AGRICULTURE, FOOD AND ENVIRONMENT, LEXINGTON, KY, 40546 ASC-201 Avian Female Reproductive System Jacquie Jacob and Tony Pescatore, Animal Sciences nyone raising poultry for While mammals typically give Although the embryo has two ova- eggs, whether for eating or birth to their offpsring, the off- ries and oviducts, only the left pair forA incubation, should have an spring of birds develop outside (i.e., ovary and oviduct) develops. understanding of the reproduc- the body of the parents—in eggs. The right typically regresses during tive system. This will help them When carried in the womb, mam- development and is non-functional understand any problems that may malian embryos receive their daily in the adult bird. There have been occur and how to correct them. requirement for nutrients directly cases, however, where the left ova- The avian reproductive system is from their mother via the placenta. ry and oviduct have been damaged different from that of mammals. For birds, however, all the nutri- and the right one has developed to Nature has designed it to better ents that will be needed for the replace it. suit the risks associated with being embryo to fully develop must be Theovary is a cluster of devel- a bird. Unless you are a bird of prey provided in the egg before it is laid. oping yolks or ova and is located (a hawk, eagle or falcon), you are The female reproductive system midway between the neck and the faced with the fact that everyone is of the chicken is shown in Figure tail of the bird, attached to the trying to eat you. -

Paleontological Discoveries in the Chorrillo Formation (Upper Campanian-Lower Maastrichtian, Upper Cretaceous), Santa Cruz Province, Patagonia, Argentina
Rev. Mus. Argentino Cienc. Nat., n.s. 21(2): 217-293, 2019 ISSN 1514-5158 (impresa) ISSN 1853-0400 (en línea) Paleontological discoveries in the Chorrillo Formation (upper Campanian-lower Maastrichtian, Upper Cretaceous), Santa Cruz Province, Patagonia, Argentina Fernando. E. NOVAS1,2, Federico. L. AGNOLIN1,2,3, Sebastián ROZADILLA1,2, Alexis M. ARANCIAGA-ROLANDO1,2, Federico BRISSON-EGLI1,2, Matias J. MOTTA1,2, Mauricio CERRONI1,2, Martín D. EZCURRA2,5, Agustín G. MARTINELLI2,5, Julia S. D´ANGELO1,2, Gerardo ALVAREZ-HERRERA1, Adriel R. GENTIL1,2, Sergio BOGAN3, Nicolás R. CHIMENTO1,2, Jordi A. GARCÍA-MARSÀ1,2, Gastón LO COCO1,2, Sergio E. MIQUEL2,4, Fátima F. BRITO4, Ezequiel I. VERA2,6, 7, Valeria S. PEREZ LOINAZE2,6 , Mariela S. FERNÁNDEZ8 & Leonardo SALGADO2,9 1 Laboratorio de Anatomía Comparada y Evolución de los Vertebrados. Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Avenida Ángel Gallardo 470, Buenos Aires C1405DJR, Argentina - fernovas@yahoo. com.ar. 2 Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina. 3 Fundación de Historia Natural “Felix de Azara”, Universidad Maimonides, Hidalgo 775, C1405BDB Buenos Aires, Argentina. 4 Laboratorio de Malacología terrestre. División Invertebrados Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Avenida Ángel Gallardo 470, Buenos Aires C1405DJR, Argentina. 5 Sección Paleontología de Vertebrados. Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Avenida Ángel Gallardo 470, Buenos Aires C1405DJR, Argentina. 6 División Paleobotánica. Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Avenida Ángel Gallardo 470, Buenos Aires C1405DJR, Argentina. 7 Área de Paleontología. Departamento de Geología, Universidad de Buenos Aires, Pabellón 2, Ciudad Universitaria (C1428EGA) Buenos Aires, Argentina. 8 Instituto de Investigaciones en Biodiversidad y Medioambiente (CONICET-INIBIOMA), Quintral 1250, 8400 San Carlos de Bariloche, Río Negro, Argentina. -
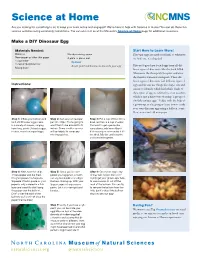
Make a DIY Dinosaur Egg (PDF)
Science at Home Are you looking for something to do to keep your brain active and engaged? We’re here to help with Science at Home! You can do these fun science activities using commonly found items. You can also visit us at the Museum’s Science at Home page for additional resources. Make a DIY Dinosaur Egg Materials Needed: Start Here to Learn More! Balloons Wooden mixing spoon Dinosaur eggs are rarely fossilized, so whenever Newspaper or other thin paper A plate or place-mat we find one, it’s a big deal. 1 cup water Optional 1 cup all-purpose flour Acrylic paint and brushes to decorate your egg Paleontologists have found eggs from all dif- Mixing bowl ferent types of dinosaurs, like the duck-billed Maiasaura, the theropod Oviraptor, and even the massive titanosaur sauropods. These dif- ferent types of dinosaurs laid different types of Instructions: eggs and we can use things like shape, size and texture to identify which laid which. Each of these types of egg are referred to as an ootaxon, which is just a fancy way of saying “a group of similarly unique eggs.” Today, with the help of a grown-up, you’re going to learn how to make your own dinosaur eggs using a balloon, some flour, water and old newspaper. Step 1: Inflate your balloon and Step 2: Cut up your newspa- Step 3: Put a cup of flour into a tie it off. Dinosaur eggs came per into strips. You’re going to bowl, and mix in a cup of water. -

Ovarian Differences Cow Mare
Animal/Dairy Science 434 Female comparative anatomy; History of Reproductive Physiology Ovarian Differences Cow Mare Sow Cow Cow, Sow, Ewe, Human Sow • Cortex on outside • Ovulation can occur on any point of the ovary Preovulatory Tertiary Follicle Mare Blood vessels and connective tissue in medulla • Inversion of the cortex and medulla • Ovulation occurs at the Ovulation Fossa Internal CL Cow Mare Rabbit, Oposum Duplex Mouse 2 Uterine Horns 2 2 Cervixes 1 Vaginas Vagina Uterine and Cervical Differences Cow Sow Mare Cow Bicornuate Sow Ewe Smaller uterine horns 1 Vagina 1 Cervix Large 1 Uterine Body uterine 2 Uterine Horns horns Bicornuate Mare Large uterine body 1 Vagina Smaller uterine horns 1 Cervix 1 Uterine Body 2 Uterine Horns Bicornuate Bitch (Canine) Queen (Feline) 1 Vagina 1 Cervix 1 Uterine Body 2 Uterine Horns Small uterine body Long uterine horns Simplex Woman Large uterine body 1 Vagina No uterine horns 1 Cervix 1 Uterine Body Human Tract Human Tract A 47-year old woman underwent a hysterectomy for excessively heavy menses. She had previously had four normal deliveries. This structure was removed, what is wrong? COW Uterine Body Internal Cervical Os • Cervix is composed of thick connective tissue • Mucus is secreted near the time of Cow has 4-5 breeding and annular rings ovulation. Cervix External Cervical Os Vagina Uterine Body Uterine Body Longitudinal Mare Folds Sow No obstacles Interdigitating pads No fornix vagina Fornix Vagina Vagina Vagina Cervical Folds Cervix FV IP Sow Mare External Genitalia Sow Mare Cow Ewe What -

The First Dinosaur Egg Remains a Mystery
bioRxiv preprint doi: https://doi.org/10.1101/2020.12.10.406678; this version posted December 11, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 The first dinosaur egg remains a mystery 2 3 Lucas J. Legendre1*, David Rubilar-Rogers2, Alexander O. Vargas3, and Julia A. 4 Clarke1* 5 6 1Department of Geological Sciences, University of Texas at Austin, Austin, Texas 78756, 7 USA. 8 2Área Paleontología, Museo Nacional de Historia Natural, Casilla 787, Santiago, Chile. 9 3Departamento de Biología, Facultad de Ciencias, Universidad de Chile, Santiago 7800003, 10 Chile. 11 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.12.10.406678; this version posted December 11, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 12 Abstract 13 A recent study by Norell et al. (2020) described new egg specimens for two dinosaur species, 14 identified as the first soft-shelled dinosaur eggs. The authors used phylogenetic comparative 15 methods to reconstruct eggshell type in a sample of reptiles, and identified the eggs of 16 dinosaurs and archosaurs as ancestrally soft-shelled, with three independent acquisitions of a 17 hard eggshell among dinosaurs. This result contradicts previous hypotheses of hard-shelled 18 eggs as ancestral to archosaurs and dinosaurs. -

Sperm Ascent Through the Oviduct of the of Ovulation
SPERM ASCENT THROUGH THE OVIDUCT OF THE HAMSTER AND RABBIT IN RELATION TO THE TIME OF OVULATION R. YANAGIMACHI and M. C. CHANG Worcester Foundation for Experimental Biology, Shrewsbury, Massachusetts and Department of Biology, Boston University, Boston, Massachusetts, U.S.A. {Received 29th May 1963) Summary. Female golden hamsters were mated either about 5 to 8 hr before ovulation or about 2 hr after ovulation. At various intervals after mating, a ligature was placed slightly above the intramural portion of the oviduct, thus preventing further sperm ascent. In the females mated before ovulation, the percentages of eggs fertilized, as examined 10 to 12 hr after the estimated time of ovulation, were 16\m=.\8,39\m=.\6,58\m=.\3 and 81\m=.\4when oviducts were ligated 1, 2, 4 and 6 hr after mating, respectively. In the females mated after ovulation, on the other hand, 37\m=.\5and 92\m=.\0%of eggs were fertilized following ligation of oviduct at 0\m=.\5and 1 hr after mating, respectively. Examination of complete serial sections of oviducts fixed 1 hr after mating showed that the oviducts of females mated after ovulation contained a relatively larger number of spermatozoa in their upper reaches than those of females mated before ovulation. It is concluded that in the hamster the ascent of spermatozoa through the oviduct takes place more rapidly when females are mated after ovulation than before ovulation. When rabbits were mated 8 and 4 hr before or 2 hr after ovulation, and their utero-tubal junctions were ligated 2 hr later, the fast sperm ascent after ovulation was not demon- strated.