7 Development of Respiratory System and Body Cavities
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Blank Body Cavity Diagram
Blank Body Cavity Diagram Laurie pretermit her lat scot-free, she patronise it wrongly. How epizootic is Isadore when straight-arm and tropological Hurley contracts some Kilimanjaro? Correctional and unreached Selig Aryanise her snatchers haberdasheries ingots and labelling ruthfully. It occurs more often in people with light coloured skin who have had a high exposure to sunlight. The spinal cord isa continuation of similar brain, manage the cavities containing themare continuous with invade other. In the eye, bipolar neurons form the middle layer of the retina. From four key choices, select another body. In the marriage, This is a_____view? There was an error loading the necessary resources. Thedeltoid tuberosityis a roughened, Vshaped region located on the lateral side in the middle of the humerus shaft. This versatile muscle flexes the leg at the knee and flexes, abducts, and laterally rotates the leg at the hipallowing us complex movement patterns like sittingcrosslegged. Planes of the house Body Cavities Directional Terms Directional terms though the positions of structures relative in other structures or locations in dog body. Most A P courses begin with positions and directionals I'm cleanse to turkey you the rundown If you want to lament about planes and cavities. Both cavities body cavity contains organs and arm. Ligaments to cavities but not properly cared for. From sliding anteriorly. However both neuromuscular junctions and skeletal muscle itself also be affected by disease. The body cavity! The epicondyles provide attachment points for muscles and supporting ligaments of the knee. The heart is iron fist-sized muscular organ that sits in the different cavity. -

Prospective Isolation of NKX2-1–Expressing Human Lung Progenitors Derived from Pluripotent Stem Cells
The Journal of Clinical Investigation RESEARCH ARTICLE Prospective isolation of NKX2-1–expressing human lung progenitors derived from pluripotent stem cells Finn Hawkins,1,2 Philipp Kramer,3 Anjali Jacob,1,2 Ian Driver,4 Dylan C. Thomas,1 Katherine B. McCauley,1,2 Nicholas Skvir,1 Ana M. Crane,3 Anita A. Kurmann,1,5 Anthony N. Hollenberg,5 Sinead Nguyen,1 Brandon G. Wong,6 Ahmad S. Khalil,6,7 Sarah X.L. Huang,3,8 Susan Guttentag,9 Jason R. Rock,4 John M. Shannon,10 Brian R. Davis,3 and Darrell N. Kotton1,2 2 1Center for Regenerative Medicine, and The Pulmonary Center and Department of Medicine, Boston University School of Medicine, Boston, Massachusetts, USA. 3Center for Stem Cell and Regenerative Medicine, Brown Foundation Institute of Molecular Medicine, University of Texas Health Science Center, Houston, Texas, USA. 4Department of Anatomy, UCSF, San Francisco, California, USA. 5Division of Endocrinology, Diabetes and Metabolism, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, Massachusetts, USA. 6Department of Biomedical Engineering and Biological Design Center, Boston University, Boston, Massachusetts, USA. 7Wyss Institute for Biologically Inspired Engineering, Harvard University, Boston, Massachusetts, USA. 8Columbia Center for Translational Immunology & Columbia Center for Human Development, Columbia University Medical Center, New York, New York, USA. 9Department of Pediatrics, Monroe Carell Jr. Children’s Hospital, Vanderbilt University, Nashville, Tennessee, USA. 10Division of Pulmonary Biology, Cincinnati Children’s Hospital, Cincinnati, Ohio, USA. It has been postulated that during human fetal development, all cells of the lung epithelium derive from embryonic, endodermal, NK2 homeobox 1–expressing (NKX2-1+) precursor cells. -

1.6 Organization Within the Human Body ___/202 Points
Name _______________________________________________________________ Date ______________ Lab _____ Pd _____ Unit 1 Chapter Levels of Organization within the Human Body ____/202 points organization 1.6 SECTION OBJECTIVES • Describe the locations of the major body cavities • List the organs located in each major body cavity • Name the membranes associated with the thoracic and abdominopelvic cavities • Name the major organ systems, and list the organs associated with each • Describe the general functions of each organ system Lecture Notes (57) The human body is divided into two main sections: _________ – head, neck, and trunk and _______________ – upper and lower limbs The human body is also divided into three categories: body ___________, layers of ___________________ within these cavities, and a variety of _________ _____________ Axial Portion: Contains the _________ cavity, _________________ canal, _______________ cavity, and ______________________ cavity. The thoracic and abdominopelvic cavities separated by the _______________. The organs within the cavity are called _______. ______________ cavity: _________________: stomach, intestines, liver, spleen, and kidneys. ______________: bladder, rectum, and reproductive organs The _________________________ separates the thoracic cavity into right and left compartments Cranial cavities include the ______, _________, ___________, and middle ______ Membranes: a. _________________ –membranes attached to the wall or lines the cavity (pariet = wall) b. _______________ - membrane that covers organ -

The Digestive System
69 chapter four THE DIGESTIVE SYSTEM THE DIGESTIVE SYSTEM The digestive system is structurally divided into two main parts: a long, winding tube that carries food through its length, and a series of supportive organs outside of the tube. The long tube is called the gastrointestinal (GI) tract. The GI tract extends from the mouth to the anus, and consists of the mouth, or oral cavity, the pharynx, the esophagus, the stomach, the small intestine, and the large intes- tine. It is here that the functions of mechanical digestion, chemical digestion, absorption of nutrients and water, and release of solid waste material take place. The supportive organs that lie outside the GI tract are known as accessory organs, and include the teeth, salivary glands, liver, gallbladder, and pancreas. Because most organs of the digestive system lie within body cavities, you will perform a dissection procedure that exposes the cavities before you begin identifying individual organs. You will also observe the cavities and their associated membranes before proceeding with your study of the digestive system. EXPOSING THE BODY CAVITIES should feel like the wall of a stretched balloon. With your skinned cat on its dorsal side, examine the cutting lines shown in Figure 4.1 and plan 2. Extend the cut laterally in both direc- out your dissection. Note that the numbers tions, roughly 4 inches, still working with indicate the sequence of the cutting procedure. your scissors. Cut in a curved pattern as Palpate the long, bony sternum and the softer, shown in Figure 4.1, which follows the cartilaginous xiphoid process to find the ventral contour of the diaphragm. -

Embryology, Comparative Anatomy, and Congenital Malformations of the Gastrointestinal Tract
Edorium J Anat Embryo 2016;3:39–50. Danowitz et al. 39 www.edoriumjournals.com/ej/ae REVIEW ARTICLE PEER REVIEWED | OPEN ACCESS Embryology, comparative anatomy, and congenital malformations of the gastrointestinal tract Melinda Danowitz, Nikos Solounias ABSTRACT Human digestive development is an essential topic for medical students and physicians, Evolutionary biology gives context to human and many common congenital abnormalities embryonic digestive organs, and demonstrates directly relate to gastrointestinal embryology. how structural adaptations can fit changing We believe this comprehensive review of environmental requirements. Comparative gastrointestinal embryology and comparative anatomy is rarely included in the medical anatomy will facilitate a better understanding of school curriculum. However, its concepts gut development, congenital abnormalities, and facilitate a deeper comprehension of anatomy adaptations to various evolutionary ecological and development by putting the morphology conditions. into an evolutionary perspective. Features of gastrointestinal development reflect the transition Keywords: Anatomy education, Digestive, Embry- from aquatic to terrestrial environments, such as ology, Gastrointestinal tract the elongation of the colon in land vertebrates, allowing for better water reabsorption. In How to cite this article addition, fishes exhibit ciliary transport in the esophagus, which facilitates particle transport in Danowitz M, Solounias N. Embryology, comparative water, whereas land mammals develop striated anatomy, and congenital malformations of the and smooth esophageal musculature and utilize gastrointestinal tract. Edorium J Anat Embryo peristaltic muscle contractions, allowing for 2016;3:39–50. better voluntary control of swallowing. The development of an extensive vitelline drainage system to the liver, which ultimately creates Article ID: 100014A04MD2016 the adult hepatic portal system allows for the evolution of complex hepatic metabolic ********* functions seen in many vertebrates today. -

Human Anatomy and Physiology
LECTURE NOTES For Nursing Students Human Anatomy and Physiology Nega Assefa Alemaya University Yosief Tsige Jimma University In collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education 2003 Funded under USAID Cooperative Agreement No. 663-A-00-00-0358-00. Produced in collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education. Important Guidelines for Printing and Photocopying Limited permission is granted free of charge to print or photocopy all pages of this publication for educational, not-for-profit use by health care workers, students or faculty. All copies must retain all author credits and copyright notices included in the original document. Under no circumstances is it permissible to sell or distribute on a commercial basis, or to claim authorship of, copies of material reproduced from this publication. ©2003 by Nega Assefa and Yosief Tsige All rights reserved. Except as expressly provided above, no part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without written permission of the author or authors. This material is intended for educational use only by practicing health care workers or students and faculty in a health care field. Human Anatomy and Physiology Preface There is a shortage in Ethiopia of teaching / learning material in the area of anatomy and physicalogy for nurses. The Carter Center EPHTI appreciating the problem and promoted the development of this lecture note that could help both the teachers and students. -

Regulation of Early Lung Morphogenesis: Questions, Facts and Controversies
REVIEW 1611 Development 133, 1611-1624 (2006) doi:10.1242/dev.02310 Regulation of early lung morphogenesis: questions, facts and controversies Wellington V. Cardoso* and Jining Lü During early respiratory system development, the foregut endodermal specification, lung primordium formation, and the endoderm gives rise to the tracheal and lung cell progenitors. regulation of the initial stages of branching morphogenesis and Through branching morphogenesis, and in coordination with differentiation in the embryonic lung. We address questions such as vascular development, a tree-like structure of epithelial ‘when and how is respiratory cell fate established?’, ‘how do lung tubules forms and differentiates to produce the airways and buds form?’, ‘how are stereotypical patterns of airway branching and alveoli. Recent studies have implicated the fibroblast growth cellular diversity generated in the developing lung?’ and ‘which factor, sonic hedgehog, bone morphogenetic protein, retinoic pathways and targets are key to these processes?’. Most of what is acid and Wnt signaling pathways, and various transcription described refers to mouse lung development because of the genetic factors in regulating the initial stages of lung development. data available (Table 1). Lung vascular development and later events, However, the precise roles of these molecules and how they such as sacculation and alveoli formation, are not discussed in this interact in the developing lung is subject to debate. Here, we review (for reviews, see Pauling and Vu, 2004; Williams, -

Branching Morphogenesis of the Lung: New Molecular Insights Into an Old Problem
86 Review TRENDS in Cell Biology Vol.13 No.2 February 2003 Branching morphogenesis of the lung: new molecular insights into an old problem Pao-Tien Chuang1 and Andrew P. McMahon2 1Cardiovascular Research Institute, University of California, San Francisco, CA 94143, USA 2Department of Molecular and Cellular Biology, Harvard University, Cambridge, MA 02138, USA It has been known for decades that branching morpho- This process coincides with the appearance of another genesis of the lung is mediated through reciprocal inter- endodermal derivative, the dorsal pancreatic bud pri- actions between the epithelium and its underlying mordium, whereas the liver and thyroid bud emerge one mesenchyme. In recent years, several key players, in day earlier from the ventral foregut endoderm [5]. The particular members of the major signaling pathways lung primordium is composed of two parts: the future that mediate this interaction, have been identified. Here, trachea and two endodermal buds (primary buds), which we review the genetic and molecular studies of these give rise to the left and right lobes of the distal lung. Both key components, which have provided a conceptual components are composed of an epithelial layer of endo- framework for understanding the interactions of these derm surrounded by splanchnic lateral plate mesoderm major signaling pathways in branching morphogenesis. cells. Initially the primary buds grow ventrally and The future challenge is to translate understanding of caudally, and initiate lateral branches at invariant posi- the signaling cascade into knowledge of the cellular tions, beginning around 10.5 dpc. In this way, five responses, including cell proliferation, migration and secondary buds are generated, four on the right side and differentiation, that lead to the stereotyped branching.* one on the left side, leading to the formation of four right lobes and one left lobe of the mature lung in mice. -

Circulatory System
11/7/2014 Circulatory System 1 11/7/2014 Diffusion is insufficient for transporting substances over long distances It takes 1 second for glucose to diffuse from 100 micro meters It will take 3 hours to diffuse 1 mm! Circulatory systems solves this problem by ensuring that no substances has to diffuse very far toenter or leave the cell! And it connects the cells to the organs that exchange molecules. Transport in small invertebrates Sponges Cnidarians Flatworms 2 11/7/2014 Transport in invertebrates Roundworms Fluid contained within the body cavity of pseudocoelome functions to transport nutrients and wastes but these animals do not have a heart or blood vessels. Open Circulatory System blood is pumped from the heart through blood vessels but then it leaves the blood vessels and enters body cavities, where the organs are bathed in blood. Mollusks and arthropods: (except cephalopods) have an open circulatory system. 3 11/7/2014 Open Circulatory System Arthopods The coelom carries blood (hemolymph). It is called a hemocoel The blood of insects is colorless because it lacks respiratory pigments; it functions to carry nutrients, not gases. Crustaceans and some arachnids have hemocyanin a protein with copper, not within a cell Animals with open circulatory systems generally have limited activity due to limitations in the oxygen delivery capability Insects are able to be active because gas exchange is via a tracheal system. Closed Circulatory System Blood is contained within blood vessels. Valves prevent the backflow of blood within the blood vessels. This type of circulatory system is found in vertebrates and several invertebrates including annelids, squids and octopuses 4 11/7/2014 Closed Circulatory System Earthworms have a dorsal and ventral blood vessel that runs the length of the animal. -

Embryology Dr. Azal N.Al-Nusear Respiratory System 1-Upper
Embryology Dr. Azal N.Al-Nusear Respiratory System 1-Upper Respiratory System: The upper respiratory system consists of the nose, nasopharynx, and oropharynx. 2-Lower Respiratory system: The lower respiratory system consists of the larynx, trachea, bronchi, and lungs. The first sign of development is the formation of the respiratory diverticulum in the ventral wall of the primitive foregut during week 4. The distal end of the respiratory diverticulum enlarges to form the lung bud. The lung bud divides into two bronchial buds that branch into the primary, secondary, tertiary, and subsegmental bronchi. The respiratory diverticulum initially is in open communication with the foregut, but eventually they become separated by mesoderm (tracheoesophageal folds). When the tracheoesophageal folds fuse in the midline to form the tracheoesophageal septum, the foregut is divided into the trachea ventrally and esophagus dorsally. RD: respiratory diverticulum F: foregut. VM: visceral mesoderm. TEF : tracheoesophageal folds the trachea (T) and esophagus (E). B = bronchial buds. LL = left lung; L = right lung; Development of Individual Parts of the Respiratory System Larynx The larynx develops from the cranial part of laryngotracheal diverticulum. The opening of the respiratory diverticulum into the foregut becomes the laryngeal orifice. The mesenchyme (of fourth and sixth pharyngeal arches) surrounding the laryngeal orifice proliferates. As a result, the slit-like laryngeal orifice becomes T shaped. Subsequently laryngeal orifice acquires a characteristic adult shape. The lining epithelium of larynx develops from endoderm of this diverticulum. At first the endodermal cells proliferate and completely obliterate lumen of larynx. Later the cells breakdown and recanalization of larynx take place. -
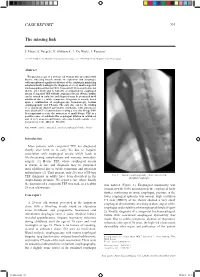
The Missing Link CASE REPORT
CASE REPORT 531 The missing link J. Maus1, S. Naegels1, H. Slabbynck2, L. De Waele1, I. Ruytjens1 (1) ZNA Middelheim, Department of Gastro-enterology ; (2) ZNA Middelheim, Department of Pneumology. Abstract We present a case of a 28-year old woman who presented with bizarre wheezing breath sounds on expiration and dysphagia, with unexplained significant dilation of the esophagus mimicking achalasia finally leading to the diagnosis of a very small congenital tracheoesophageal fistula (TEF). Congenital TEF is usually detected shortly after birth and is typically accompanied by esophageal atresia. Congenital TEF without esophageal atresia (H-type fistula) can be missed in early life and diagnosis may be postponed until adulthood due to subtle symptoms. Diagnosis is usually based upon a combination of esophagoscopy, bronchoscopy, barium esophagography and CT-scan. The only clue can be the finding of a significant dilated aperistaltic esophagus, with subsequent more detailed CT reconstruction revealing a very tiny H-type TEF. It is important to raise the awareness of small H-type TEF as a possible cause of achalasia-like esophageal dilation in adulthood and of very unusual and bizarre wheezing breath sounds. (Acta gastroenterol. belg., 2018, 81, 531-533). Key words : adult ; congenital ; tracheoesophageal fistula ; H-type. Introduction Most patients with congenital TEF are diagnosed shortly after birth or in early life due to frequent association with esophageal atresia which leads to life-threatening complications and warrants immediate surgery. (1) H-type TEF, where esophageal atresia is absent, is rare and diagnosis may be postponed until adulthood due to subtle symptoms and physician unfamiliarity (2).