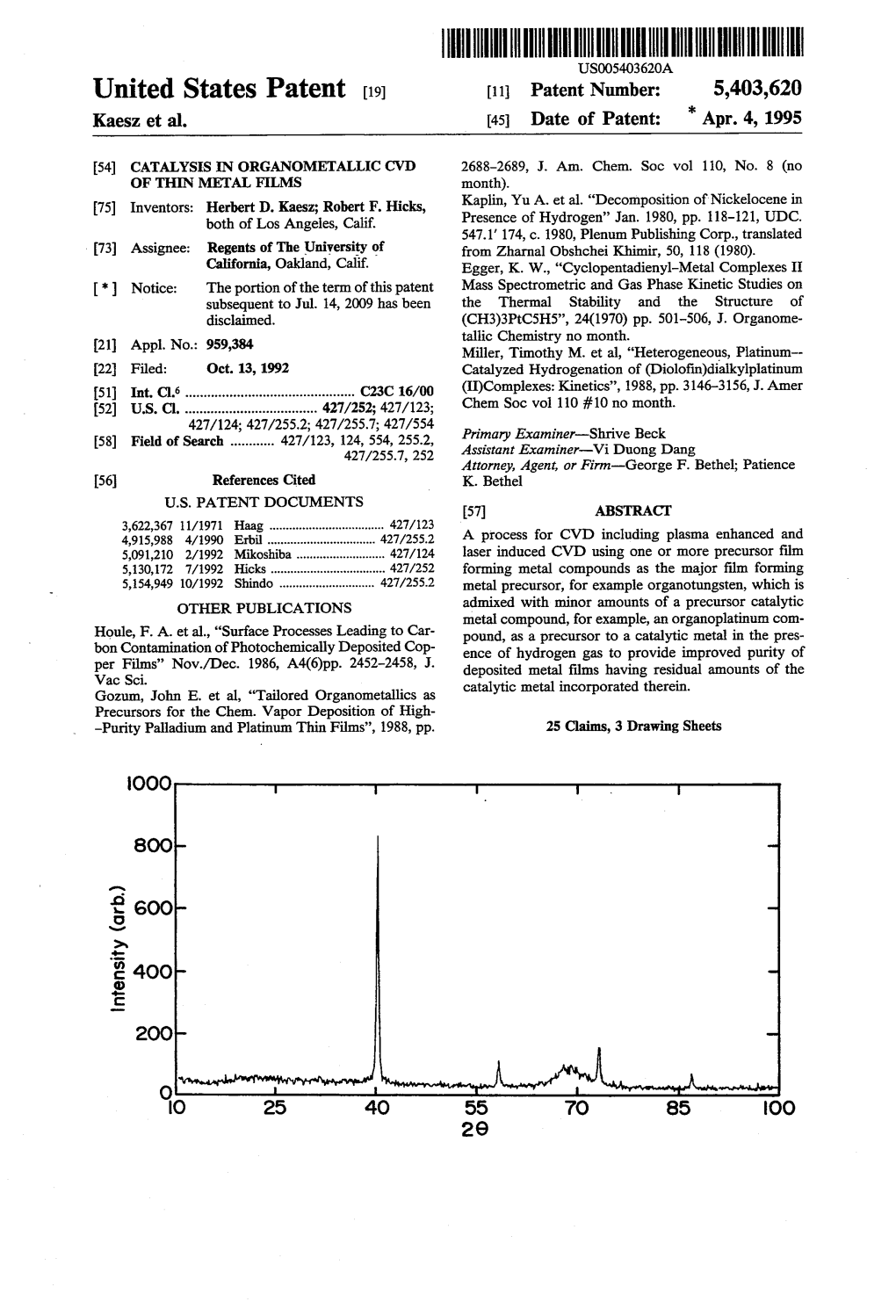
United States Patent (19) Apr. 4, 1995
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spark Plasma Sintering of Tantalum Carbide and Graphene Reinforced Tantalum Carbide Ceramic Composites
SPARK PLASMA SINTERING OF TANTALUM CARBIDE AND GRAPHENE REINFORCED TANTALUM CARBIDE COMPOSITES By AJITH KUMAR KALLURI Bachelor of Science in Mechanical Engineering VIT University Vellore, India 2010 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE December, 2012 SPARK PLASMA SINTERING OF TANTALUM CARBIDE AND GRAPHENE REINFORCED TANTALUM CARBIDE COMPOSITES Thesis Approved: Dr. Sandip P. Harimkar Thesis Adviser Dr. A. Kaan Kalkan Dr. Raman P. Singh ii ACKNOWLEDGEMENTS I would like to thank my advisor Dr. Sandip P. Harimkar for his kind advise, guidance, and patience throughout my masters. I would also be grateful to Dr. A. Kaan Kalkan and Dr. Raman P. Singh for being my committee members and their valuable suggestions throughout my thesis. I would like to convey my deepest sense of gratitude to Ashish Singh for his valuable suggestions, support, and care, Sriharsha Karumuri for his encouragement, moral support and Sudheer Bandla for his help with my thesis. I could not have completed my thesis without their help. It will take a life time to forget the precious time, love and support which they gave when in need. I would also like to thank Amy Aurilio for her help with my thesis write up. Last but not the least; I am very grateful to my parents (Ramesh Babu Kalluri and Sarada Gogineni) and my sister (Divya Sree Kalluri) for their love, support and encouragement throughout my career. Heartful thanks to my uncle Dr. Sridhar Gogineni, his wife Manasa Gogineni, my sister Neelima Kalluri and my very lovable niece Sreeja Gogineni who never let me miss home. -

A Case for Capacitor Grade Sintered Tantalum
Bull. Mater. Sci., Vol. 28, No. 4, July 2005, pp. 305–307. © Indian Academy of Sciences. Powder metallurgical processing and metal purity: A case for capacitor grade sintered tantalum G S UPADHYAYA Department of Materials and Metallurgical Engineering, Indian Institute of Technology, Kanpur 208 016, India Abstract. The paper reviews the role of sintered tantalum as volumetric efficient electrical capacitor. Powder characteristics and sintering aspects are discussed. The role of impurities in influencing the electrical properties has been described. Today’s driving force behind the Ta market is the use of surface mounted versions known as chip types, for applications requiring a wide range of operational temperature, such as automotive electronics. Keywords. Tantalum powder; sintering; capacitor. 1. Introduction tantalum powder. The pellet, with an attached tantalum lead wire, is electrochemically oxidized to grow a thin Many products and devices are being manufactured through layer of insulating tantalum oxide on the surface of the powder metallurgy route, because of many associated tantalum. Next, the anodized pellet is impregnated with advantages (Upadhyaya 1997). The purity of the starting manganese nitrate which is then thermally decomposed to metal or ceramic powder is of significance in controlling leave or deposit semiconducting manganese dioxide on the microstructure/properties/processing and performance the tantalum oxide. These processes create the conduc- of such products. The major methods of production of tor (Ta)/insulator(Ta2O5)/conductor(MnO2) configuration metal powders are: chemical, physical and mechanical. needed for a capacitor. Finally, the unit is encapsulated Tantalum is used mainly as a corrosion resistant metal usually in the chip configuration. -

Chemical Vapor Deposition of Tac/Sic on Graphite Tube and Its Ablation and Microstructure Studies
coatings Article Chemical Vapor Deposition of TaC/SiC on Graphite Tube and Its Ablation and Microstructure Studies Suresh Kumar 1,*, Samar Mondal 2, Anil Kumar 2, Ashok Ranjan 1 and Namburi Eswara Prasad 1 1 Directorate of Ceramics and CMCs, Defence Materials & Stores Research and Development Establishment, Defence Research and Development Organization, Kanpur 208013, India; [email protected] (A.R.); [email protected] (N.E.P.) 2 Advanced Systems Laboratory, Defence Research and Development Organization, Hyderabad 500058, India; [email protected] (S.M.); [email protected] (A.K.) * Correspondence: [email protected]; Tel.: +91-512-240-3693 Received: 15 June 2017; Accepted: 10 July 2017; Published: 13 July 2017 Abstract: Tantalum carbide (TaC) and silicon carbide (SiC) layers were deposited on a graphite tube using a chemical vapor deposition process. Tantalum chloride (TaCl5) was synthesized in ◦ situ by reacting tantalum chips with chlorine at 550 C. TaC was deposited by reacting TaCl5 with ◦ ◦ CH4 in the presence of H2 at 1050–1150 C and 50–100 mbar. SiC was deposited at 1000 C using methyl-tri-chloro-silane as a precursor at 50 mbar. At 1150 ◦C; the coating thickness was found to be about 600 µm, while at 1050 ◦C it was about 400 µm for the cumulative deposition time of 10 h. X-ray diffraction (XRD) and X-ray Photo-Electron Spectroscopy (XPS) studies confirmed the deposition of TaC and SiC and their phases. Ablation studies of the coated specimens were carried out under oxyacetylene flame up to 120 s. The coating was found to be intact without surface cracks and with negligible erosion. -

Thermal Analysis of Tantalum Carbide-Hafnium Carbide Solid Solutions from Room Temperature to 1400 ◦C
coatings Article Thermal Analysis of Tantalum Carbide-Hafnium Carbide Solid Solutions from Room Temperature to 1400 ◦C Cheng Zhang, Archana Loganathan, Benjamin Boesl and Arvind Agarwal * Plasma Forming Laboratory, Department of Mechanical and Materials Engineering, Florida International University, Miami, 33139 FL, USA; czhan009@fiu.edu (C.Z.); aloga006@fiu.edu (A.L.); bboesl@fiu.edu (B.B.) * Correspondence: agarwala@fiu.edu; Tel.: +1-305-348-1701 Received: 5 June 2017; Accepted: 25 July 2017; Published: 28 July 2017 Abstract: The thermogravimetric analysis on TaC, HfC, and their solid solutions has been carried out in air up to 1400 ◦C. Three solid solution compositions have been chosen: 80TaC-20 vol % HfC (T80H20), 50TaC-50 vol % HfC (T50H50), and 20TaC-80 vol % HfC (T20H80), in addition to pure TaC and HfC. Solid solutions exhibit better oxidation resistance than the pure carbides. The onset of oxidation is delayed in solid solutions from 750 ◦C for pure TaC, to 940 ◦C for the T50H50 sample. Moreover, T50H50 samples display the highest resistance to oxidation with the retention of the initial carbides. The oxide scale formed on the T50H50 sample displays mechanical integrity to prevent the oxidation of the underlying carbide solid solution. The improved oxidation resistance of the solid solution is attributed to the reaction between Ta2O5 and HfC, which stabilizes the volume changes induced by the formation of Ta2O5 and diminishes the generation of gaseous products. Also, the formation of solid solutions disturbs the atomic arrangement inside the lattice, which delays the reaction between Ta and O. Both of these mechanisms lead to the improved oxidation resistances of TaC-HfC solid solutions. -

A Comparison Among Pressureless Sintered Ultra-Refractory Carbides
Hindawi Publishing Corporation Advances in Materials Science and Engineering Volume 2010, Article ID 835018, 11 pages doi:10.1155/2010/835018 Research Article Sintering Behavior, Microstructure, and Mechanical Properties: A Comparison among Pressureless Sintered Ultra-Refractory Carbides Laura Silvestroni and Diletta Sciti CNR-ISTEC, Institute of Science and Technology for Ceramics, Via Granarolo 64, I-48018 Faenza, Italy Correspondence should be addressed to Laura Silvestroni, [email protected] Received 12 July 2010; Accepted 10 October 2010 Academic Editor: Paul Munroe Copyright © 2010 L. Silvestroni and D. Sciti. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Nearly fully dense carbides of zirconium, hafnium, and tantalum were obtained by pressureless sintering at 1950 ◦C with the addition of 5–20 vol% of MoSi2. Increasing the amount of sintering aid, the final density increased too, thanks to the formation of small amounts of liquid phase constituted by M-Mo-Si-O-C, where M is either Zr, Hf, or Ta. The matrices of the composites obtained with the standard procedure showed faceted squared grains; when an ultrasonication step was introduced in the powder treatment, the grains were more rounded and no exaggerated grains growth occurred. Other secondary phases observed in the microstructure were SiC and mixed silicides of the transition metals. Among the three carbides prepared by pressurless sintering, TaC-based composites had the highest mechanical properties at room temperature (strength 590 MPa, Young’s modulus 480 GPa, toughness 3.8 MPa·m1/2). -

Study of Growth Parameters for Refractory Carbide Single Crystals
Final Report I March 7, 1964 to June 30, 1967 STUDY OF GROWTH PARAMETERS FOR REFRACTORY CARBIDE SINGLE CRYSTALS Prepared for: OFFICE OF RESEARCH GRANTS AND CONTRACTS CODE SC NATIONAL AERONAUTICS AND SPACE ADMINISTRATION WASHINGTON, D.C. 20546 CONTRACT NASr-49(19) 69: R. W. BARTLETT AE 4D F. A. HALDEN SRI Proie ct FMU-48 92 N I CT June 30, 7967 Final Report March 7, 7964 to June 30, 7967 "g_".,I ?)STUDY OF GROWTH PARAMETERS FOR REFRACTORY CARBIDE SINGLE CRYSTALS Prepared for: OFFICE OF RESEARCH GRANTS AND CONTRACTS CODE SC NA TI ONAL A ERONAUTI CS AND SP AC E ADMl N IST RAT I ON WASHINGTON, D.C. 20546 CONTRACT NASr-49(19) "I * i>L ! . W. BARTLETT AND F. A. HALDEN S R I Project FMU-4892 Approved: A. E. GORUM, ASSOCIATE EXECUTIVE DIRECTOR MA TERl AL SCIENCES LABOR ATORl ES FOREWORD The research described in this report was performed at Stanford Research Institute under Contract NASr-49(19) with the Office of Research Grants and Contracts, National Aeronautics and Space Admin- istration. Dr. H. B. Probst was the Project Monitor. The assistance of Mr. James J. Gangler is gratefully acknowledged. This report covers work conducted between 1 March 1964 and 30 June 1967. The principal investigators were F. A. Halden and R. W. Bartlett. The authors wish to acknowledge the assistance of J. W. Fowler, melt growth; W. E. Nelson, solution growth; J. B. Saunders, X-ray diffraction; H. J. Eding, vaporization and stoichi- ometry calculations; and C. W. Smith, sound velocity measurements. iii ABSTRACT The feasibility of growing single crystals of the most refractory metal carbides from their melts and from liquid metal solutions was investigated. -

Cemented Carbidescarbides Forfor Powderpowder Metalmetal Toolingtooling Applicationsapplications
AdvancedAdvanced CementedCemented CarbidesCarbides forfor PowderPowder MetalMetal ToolingTooling ApplicationsApplications Dr. Leonid I. Frayman Chief Metallurgist 41th Anniversary 41th Anniversary Presented at MPIF “PM Compacting & Tooling” 1968-2009 1968-2009 Seminar (October 27-28, 2009. Cleveland, OH.) CARBIDES?… ……WWhhaatt ddoo wwee kknnooww aabboouutt tthheemm?? Agenda: What is a cemented carbide? Why do we use cemented carbide in PM? What advancements have been made for PM tooling applications in: - carbide processing and manufacturing? - carbide grade development? - carbide failure analysis and troubleshooting? WhatWhat isis CementedCemented Carbide?Carbide? Definition: Cemented Carbide is a composite material of a soft binder metal usually either Cobalt (Co) or Nickel (Ni) or Iron (Fe) or a mixture thereof and hard carbides like WC (Tungsten Carbide), Mo2C (Molybdenum Carbide), TaC (Tantalum Carbide), Cr3C2 (Chromium Carbide), VC (Vanadium Carbide), TiC (Titanium Carbide), etc. or their mixes. Carbides: Selected Mechanical Properties. Carbide Vickers (HV) Hardness @ Various Rockwell Hardness Ultimate Compressive Transverse Modulus of Formula Temperatures, oC (oF) @ Room Strength, Rupture Strength, Elasticity, Temperature, MPa (ksi) MPa (ksi) GPa (106 ksi) 20 oC (78 oF) 730 oC (1350 oF) HRa TiC* 2930 640 93 1330-3900 (193-522) 280-400 (40.6-58.0) 370 (52.9) HfC* 2860 - 84 - - - VC* 2800 250 83 620 (89.9) 70 (10.1) 360 (51.4) NbC 2400 350 83 1400 (203) - 270 (38.5) * TaC* 1570 800 82 - - 470 (68,2) - - 81 100 (14.5) 170-380 ((24.7-55.1) 280 (40.0) Cr3C2* - - 2700 (392) 50 (7.3) Mo2C* 74 375 (53.6) WC* 2400 280 81 2700-3600 (392-522) 530-560 (76.9-81.2) 665 (95) *NOTE: TiC-Titanium Carbide; HfC-Hafnium Carbide; VC-Vanadium Carbide; NbC-Niobium Carbide; TaC-Tantalum Carbide; Cr3C2 - Chromium Carbide; Mo2C - Molybdenum Carbide; WC-Tungsten Carbide. -

Processing Development of 4Tac-Hfc and Related Carbides and Borides for Extreme Environments Osama Gaballa Gaballa Iowa State University
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2012 Processing development of 4TaC-HfC and related carbides and borides for extreme environments Osama Gaballa Gaballa Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Mechanics of Materials Commons Recommended Citation Gaballa, Osama Gaballa, "Processing development of 4TaC-HfC and related carbides and borides for extreme environments" (2012). Graduate Theses and Dissertations. 12635. https://lib.dr.iastate.edu/etd/12635 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Processing development of 4TaC-HfC and related carbides and borides for extreme environments by Osama Gaballa Bahig Gaballa A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Major: Materials Science and Engineering Program of Study Committee: Alan M. Russell, Major Professor Vitalij Pecharsky Scott Chumbley Kristen Constant Sriram Sundararajan Iowa State University Ames, Iowa 2012 Copyright © Osama Gaballa Bahig Gaballa, 2012. All rights reserved. ii Table of Contents Abstract 1 Chapter 1: Introduction 4 1.1 Aluminum Silicon -

Safety Data Sheet
SAFETY DATA SHEET According to JIS Z 7253:2019 Revision Date 26-Apr-2021 Version 2.01 Section 1: PRODUCT AND COMPANY IDENTIFICATION Product name Tantalum Carbide Product code 201-12562 Manufacturer FUJIFILM Wako Pure Chemical Corporation 1-2 Doshomachi 3-Chome Chuo-ku, Osaka 540-8605, Japan Phone: +81-6-6203-3741 Fax: +81-6-6203-5964 Supplier FUJIFILM Wako Pure Chemical Corporation 1-2 Doshomachi 3-Chome, Chuo-ku, Osaka 540-8605, Japan Phone: +81-6-6203-3741 Fax: +81-6-6203-2029 Emergency telephone number +81-6-6203-3741 / +81-3-3270-8571 Recommended uses and For research use only restrictions on use Section 2: HAZARDS IDENTIFICATION GHS classification Classification of the substance or mixture Not a hazardous substance or mixture according to the Globally Harmonized System (GHS) Pictograms Signal word none Hazard statements Not a hazardous substance or mixture according to the Globally Harmonized System (GHS) Precautionary statements-(Prevention) • Not applicable Precautionary statements-(Response) • Not applicable Precautionary statements-(Storage) • Not applicable Precautionary statements-(Disposal) • Not applicable Others Other hazards Not available Section 3: COMPOSITION/INFORMATION ON INGREDIENTS Single Substance or Mixture Substance Formula TaC Chemical Name Weight-% Molecular weight ENCS ISHL No. CAS RN Tantalum Carbide 6.1 (as C) 192.96 (1)-616 (1)-616 12070-06-3 Impurities and/or Additives: Not applicable __________________________________________________________________________________________ Page 1 / 5 W01W0120-1256 JGHEEN Tantalum Carbide Revision Date 26-Apr-2021 __________________________________________________________________________________________ Section 4: FIRST AID MEASURES Inhalation Remove to fresh air. If symptoms persist, call a physician. Skin contact Wash off immediately with soap and plenty of water. -

Category 1—Page 1
Commerce Control List Supplement No. 1 to Part 774 Category 1—page 1 CATEGORY 1 - SPECIAL MATERIALS AND to the ITAR” (see 22 CFR parts 120 through RELATED EQUIPMENT, CHEMICALS, 130, including USML Category XXI). (2) “MICROORGANISMS,” AND “TOXINS” See also 1C009. Related Definitions: N/A Note: The Food and Drug Administration Items: (FDA) and the Drug Enforcement Administration (DEA) may control exports of items subject to the a. Seals, gaskets, sealants or fuel bladders, EAR and on the Commerce Control List. BIS “specially designed” for “aircraft” or aerospace provides cross references to these other agency use, made from more than 50% by weight of any controls for convenience only. Therefore, please of the materials controlled by 1C009.b or consult relevant FDA and DEA regulations for 1C009.c; guidance related to the item you wish to export and do not rely solely on the EAR for information b. [Reserved] about other agency export control requirements. See Supplement No. 3 to part 730 (Other U.S. Government Departments and Agencies with 1A002 “Composite” structures or laminates, Export Control Responsibilities) for as follows (see List of Items Controlled). more information. License Requirements A. “END ITEMS,” “EQUIPMENT,” Reason for Control: NS, NP, AT “ACCESSORIES,” “ATTACHMENTS,” “PARTS,” “COMPONENTS,” AND Control(s) Country Chart “SYSTEMS” (See Supp. No. 1 to part 738) 1A001 “Parts” and “components” made from NS applies to entire entry NS Column 2 fluorinated compounds, as follows (see List of NP applies to 1A002.b.1 in NP Column 1 Items Controlled). the form of tubes with an inside diameter between 75 License Requirements mm and 400 mm AT applies to entire entry AT Column 1 Reason for Control: NS, AT Reporting Requirements Country Chart Control(s) (See Supp. -

Angstrom Sciences PVD Materials
40 S. Linden Street, Duquesne, PA 15110 USA Tel: (412) 469-8466 Fax: (412) 469-8511 www.angstromsciences.com PVD Materials Target Sputtering Targets, Pellets, Bonding Services Slugs, Starter Charges, Crucibles Recycling of precious metal All Configurations of Clips, scrap Rod, Tube, pieces and Wire Pure Elements / Noble Metals Aluminum, (Al) Gadolinium, (Gd) Nickel, (Ni) Tantalum, (Ta) Antimony, (Sb) Gallium, (Ga) Niobium, (Nb) Tellurium, (Te) Beryllium, (Be) Germanium, (Ge) Osmium, (Os) Terbium, (Tb) Bismuth, (Bi) Gold, (Au) Palladium, (Pd) Tin, (Sn) Boron, (B) Hafnium, (Hf) Platinum, (Pt) Titanium, (Ti) Cadmium, (Cd) Indium, (In) Praseodymium, (Pr) Tungsten, (W) Calcium, (Ca) Iridium, (Ir) Rhenium, (Re) Vanadium, (V) Carbon, (C) Iron, (Fe) Rhodium, (Rh) Ytterbium, (Yb) Cerium, (Ce) Lanthanum, (La) Ruthenium, (Ru) Yttrium, (Y) Chromium, (Cr) Lead, (Pb) Samarium, (Sm) Zinc, (Zn) Cobalt, (Co) Magnesium, (Mg) Selenium, (Se) Zirconium, (Zr) Copper, (Cu) Manganese, (Mn) Silicon, (Si) Erbium, (Er) Molybdenum, (Mo) Silver, (Ag) Precious Metal Alloys Gold Antimony, (Au/Sb) Gold Zinc, (Au/Zn) Gold Boron, (Au/B) Palladium Manganese, (Pd/Mn) Gold Copper, (Au/Cu) Palladium Nickel, (Pd/Ni) Gold Germanium, (Au/Ge) Platinum Palladium, (Pt/Pd) Gold Nickel, (Au/Ni) Palladium Rhenium, (Pd/Re) Gold Nickel Indium, (Au/Ni/In) Platinum Rhodium, (Pt/Rh) Gold Palladium, (Au/Pd) Silver Copper, (Ag/Cu) Gold Silicon, (Au/Si) Silver Gallium, (Ag/Ga) Gold Silver Platinum, (Au/Ag/Pt) Silver Gold, (Ag/Au) Gold Tantalum, (Au/Ta) Silver Palladium, (Ag/Pd) Gold Tin, -

Precursor Chemistry of Tantalum and Niobium Nitride for MOCVD and ALD Applications
Precursor Chemistry of Tantalum and Niobium Nitride for MOCVD and ALD Applications Dissertation Arne Baunemann Precursor Chemistry of Tantalum and Niobium Nitride for MOCVD and ALD Applications Dissertation zur Erlangung der Doktorwürde der Fakultät für Chemie der Ruhr-Universität Bochum vorgelegt von Diplom-Chemiker Arne Baunemann Referenten Prof. Dr. Roland A. Fischer Prof. Dr. William S. Sheldrick II Die vorliegende Arbeit entstand in der Zeit von Oktober 2003 bis Oktober 2006 am Lehrstuhl für Anorganische Chemie II der Ruhr-Universität Bochum Meinem Mentor und Betreuer, Professor Dr. Roland A. Fischer möchte ich an dieser Stelle meinen außerordentlichen Dank zuteil werden lassen. Ohne seine inspirierende Vorlesung im ersten Semester hätte ich nicht den Weg in die Chemie gefunden. Während meines gesamten Studiums hat er mir jederzeit mit Rat und Tat zur Seite gestanden und mich in allen Plänen - ohne Einschränkungen - unterstützt. Diese Erfahrungen haben mich zutiefst für mein weiteres Leben geprägt. III “Contradictions do not exist. Whenever you think that you are facing a contradiction, check your premises. You will find that one of them is wrong.” (Ayn Rand in “Atlas Shrugged”, 1957) Meinen Eltern und Carmen IV Acknowledgements Danksagung Juniorprof. Dr. Anjana Devi Anjana und Harish danke ich außerordentlich für die & Dr. Harish Parala Unterstützung in Form von konstruktiver Kritik in vielen Bereichen und der Unterstützung bei XRD und TG/DTA Messungen. Dr. Christian Gemel Christian danke ich für den wertvollen chemischen Input, den er mir in den letzten Jahren gegeben hat. Marie-Kathrin Schröter Nana danke ich für die Unterstützung und den gemeinsamen Spaß in den letzten acht Jahren, die wir fast tagein-tagaus miteinander verbracht haben.