Rare Earths-2017.Pmd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Use of Phosphate Solubilizing Bacteria to Leach Rare Earth Elements from Monazite-Bearing Ore
Minerals 2015, 5, 189-202; doi:10.3390/min5020189 OPEN ACCESS minerals ISSN 2075-163X www.mdpi.com/journal/minerals Article Use of Phosphate Solubilizing Bacteria to Leach Rare Earth Elements from Monazite-Bearing Ore Doyun Shin 1,2,*, Jiwoong Kim 1, Byung-su Kim 1,2, Jinki Jeong 1,2 and Jae-chun Lee 1,2 1 Mineral Resources Resource Division, Korea Institute of Geoscience and Mineral Resources (KIGAM), Gwahangno 124, Yuseong-gu, Daejeon 305-350, Korea; E-Mails: [email protected] (J.K.); [email protected] (B.K.); [email protected] (J.J.); [email protected] (J.L.) 2 Department of Resource Recycling Engineering, Korea University of Science and Technology, Gajeongno 217, Yuseong-gu, Daejeon 305-350, Korea * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +82-42-868-3616. Academic Editor: Anna H. Kaksonen Received: 8 January 2015 / Accepted: 27 March 2015 / Published: 2 April 2015 Abstract: In the present study, the feasibility to use phosphate solubilizing bacteria (PSB) to develop a biological leaching process of rare earth elements (REE) from monazite-bearing ore was determined. To predict the REE leaching capacity of bacteria, the phosphate solubilizing abilities of 10 species of PSB were determined by halo zone formation on Reyes minimal agar media supplemented with bromo cresol green together with a phosphate solubilization test in Reyes minimal liquid media as the screening studies. Calcium phosphate was used as a model mineral phosphate. Among the test PSB strains, Pseudomonas fluorescens, P. putida, P. rhizosphaerae, Mesorhizobium ciceri, Bacillus megaterium, and Acetobacter aceti formed halo zones, with the zone of A. -

HIGHLIGHTS Special Topics: Technical Insights: News Throughout the Region: NEWS INDIA& MIDDLE EAST
OCTOBER / NOVEMBER HIGHLIGHTS 2020 - ISSUE 11 Special Topics: SPOTLIGHT ON Johan Sverdrup: a Norwegian megaproject 7 JD Jones poised for explosive Regional Focus: Karnataka 11 growth Interview: IMI Regional President Mr Tarak Chhaya 15 With strong leadership, a clear strategy, and the ability to quickly adapt to changing circumstances, JD Jones Technical Insights: is a real force in the manufacture Compliant valve stem seals reduce emissions 13 and supply of fluid sealing products. The product range – including gland Could hydrogen be the ideal green fuel? 17 packings, seals, compression packings, PFTE products, etc – finds widespread Automation upgrade for Assam’s tea gardens 22 use in a diverse mix of industries around the world. The company has INDIA & MIDDLE EAST News throughout the Region: also forged win-win partnerships with many leading valvemakers, as Valve 2, 6, 9, 10, 12, 16, 18, 20, 24 World India & Middle East discovered when speaking recently to CEO The insiders guide to flow control in India, Iran, Bahrain, Egypt, Kuwait, Oman, Azerbaijan, Jordan, Kazakhstan, Qatar, Saudi Arabia, Sri Lanka, UAE, Bangladesh Mr. Ashish Bajoria. “When Covid-19 struck India in February we of course complied fully Specialist in : with the lockdown. But we did not Multiport allow ourselves to be cowed down by the situation, far from it. We continued Ball Valves to support our clients to the very best Distributors of our ability. Moreover, we used this Wanted period as the ideal opportunity to brainstorm about new markets. I am therefore delighted to say that since Available at February 2020 we have in fact opened JD CONTROLS up four new product verticals.” www.multiportballvalves.com Continued on page 4 NEWS NFC’s plans for a new facility at Kota by 2022 RIL - 1st Indian company to hit $200bn mcap A new facility of the city-based dles, which will be produced at Read more on page 2. -

COLUMBIUM - and RARE-EARTH ELEMENT-BEARING DEPOSITS at BOKAN MOUNTAIN, SOUTHEAST ALASKA by J
COLUMBIUM - AND RARE-EARTH ELEMENT-BEARING DEPOSITS AT BOKAN MOUNTAIN, SOUTHEAST ALASKA By J. Dean Warner and James C. Barker Alaska Field Operations Center * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * OFR - 33-89 UNITED STATES DEPARTMENT OF THE INTERIOR Manuel Lujan, Jr., Secretary BUREAU OF MINES T.S. Ary, Director TABLE OF CONTENTS Page Abstract ........................................................ 1 Introduction ....................... 2 Acknowledgments ................................................. 4 Location, access, and physiography .............................. 5 History and production ............. 5 Geologic setting .................................. 9 Trace element analyses of the peralkaline granite .............. 11 Nature of Bureau investigations ................................. 13 Sampling methods and analytical techniques .................... 13 Analytical interference, limitations, and self-shielding ...... 13 Resource estimation methods ................................... 15 Prospect evaluations .......................................... 16 Shear zones and fracture-controlled deposits .................... 18 Ross Adams mine ................. 18 Sunday Lake prospect.......................................... 21 I and L No. 1 and Wennie prospects ............................ 27 Other Occurrences ............................................. 31 Altered peralkaline granite ................................. 31 Dotson shear zone ........................................... 35 Resources .................................................. -

(12) Patent Application Publication (10) Pub. No.: US 2014/0166788 A1 Pearse Et Al
US 2014O166788A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0166788 A1 Pearse et al. (43) Pub. Date: Jun. 19, 2014 (54) METHOD AND SYSTEM FOR MAGNETIC (52) U.S. Cl. SEPARATION OF RARE EARTHIS CPC. B03C I/02 (2013.01); B02C 23/08 (2013.01); B02C23/20 (2013.01) (76) Inventors: Gary Pearse, Ottawa (CA); Jonathan USPC .......... 241/20: 209/212: 209/214; 241/24.14; Borduas, Montreal (CA); Thomas 241/21 Gervais, Montreal (CA); David Menard, Laval (CA); Djamel Seddaoui, Repentigny (CA); Bora Ung, Quebec (CA) (57) ABSTRACT (21) Appl. No.: 13/822,363 A system and a method for separating rare earth element (22) PCT Filed: Aug. 15, 2012 compounds from a slurry of mixed rare earth element com pounds, comprising flowing the slurry of mixed rare earth (86). PCT No.: PCT/CA12/50.552 element compounds through at least a first channel rigged S371 (c)(1), with at least a first magnet along a length thereof and con (2), (4) Date: May 17, 2013 nected to at least a first output channel at the position of the Publication Classification magnet, and retrieving individual rare earth element com pounds and/or groups of rare earth element compounds, sepa (51) Int. Cl. rated from the slurry as they are selectively attracted by the BO3C I/02 (2006.01) magnet and directed in the corresponding output channel B2C 23/20 (2006.01) according to their respective ratio of magnetic Susceptibility B2C 23/08 (2006.01) (AX) to specific density (Ap). Ferromagnetic particles are dia?pararnegnetic particles Submitted to a magnetic torque remain relatively unaffected by the torque As a result they roll left \\ Carried right by the slow rotating drum Patent Application Publication Jun. -

WO 2011/156817 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date Χ t it o n 1 15 December 2011 (15.12.2011) WO 2011/156817 Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C02F1/58 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, (21) International Application Number: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, PCT/US20 11/040214 DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, 13 June 201 1 (13.06.201 1) KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (25) Filing Language: English NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (26) Publication Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 61/354,031 11 June 2010 ( 11.06.2010) (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant (for all designated States except US): MOLY- GM, KE, LR, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, CORP MINERALS LLC [US/US]; 561 Denver Tech ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, Center Pkwy, Suite 1000, Greenwood Village, CO 801 11 TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, (US). -

Some Uses of Mischmetall in Organic Synthesis
REVIEW 9 Mischmetall in organic synthesis Organic synthesis Organic synthesis New from Acros Organics Marie-Isabelle Lannou, Florence Hélion and Jean-Louis Namy Laboratoire de Catalyse Moléculaire, associé au CNRS, ICMO, Bat 420, Université de Paris-Sud, 91405, Orsay, France Some uses of mischmetall in organic synthesis ∗ Marie-Isabelle Lannou, Florence Hélion and Jean-Louis Namy Laboratoire de Catalyse Moléculaire, associé au CNRS, ICMO, Bat 420, Université Paris-Sud, 91405, Orsay, France. Abstract: Mischmetall, an alloy of the light lanthanides, has been used in a variety of organic reactions, either as a coreductant in samarium(II)-mediated reactions (Barbier and Grignard-type reactions, pinacolic coupling reactions) or as the promoter of Reformatsky-type reactions. It has been also employed as the starting material for easy syntheses of lanthanide trihalides, the reactivity of which has been explored in Imamoto and Luche-Fukuzawa reactions and in Mukaiyama aldol reactions. Keywords: mischmetall, samarium, lanthanide, catalysis, organic reactions Introduction The use of rare earth compounds in organic chemistry has grown considerably during the past twenty years. Mainly samarium, cerium, lanthanum, ytterbium, neodymium, dysprosium, lutetium, scandium and yttrium metals and derivatives have been studied. These elements clearly differ in terms of reactivity. For instance, samarium(II) compounds can be used as powerful reductants whereas cerium(IV) ones are strong oxidants, some scandium and ytterbium(III) derivatives are very efficient catalysts in a variety of reactions and chemistry of lanthanum metal shows special features. We thought however that in many reactions, a mixture of lanthanide could be used instead of individual element without significant change in results. -

Management of the Indian Nuclear Fuel Fabrication Facilities During COVID – 19 Pandemic
Department of Atomic Energy Nuclear Fuel Complex Hyderabad – Pazhayakayal – Kota Online Webinar Management of the Indian Nuclear Fuel Fabrication Facilities during COVID – 19 Pandemic Dr. Dinesh Srivastava Distinguished Scientist Chairman & Chief Executive IAEA Online Webinar: Maintaining Nuclear Safety of Nuclear Fuel Cycle Facilities during Pandemic 1 Outline Introduction Fulfillment of NFC commitment Infrastructure development Covid-19 protection plan during production New norm post covid-19 Summary. IAEA Online Webinar: Maintaining Nuclear Safety of Nuclear Fuel Cycle Facilities during Pandemic 2 Fuel Bundles Manufactured at NFC 220 MWe PHWR 540 MWe PHWR 160 MWe BWR (RAPS 3&4) (TAPS 3&4) (TAPS 1&2) 19 Element Bundle 37 Element Bundle 6 × 6 BWR Bundle 16.5 kg in Weight 23.8 kg in Weight 203 kg in Weight IAEA Online Webinar: Maintaining Nuclear Safety of Nuclear Fuel Cycle Facilities during Pandemic 3 PHWR Fuel Manufacturing Process MDU / SDU / Washed and Dissolution HTUP / UOC Dried Frit Dissolution Solvent Extraction Solvent Extraction Precipitation Precipitation Drying Filtration Calcination Drying Reduction Calcination Stabilization Grinding Nuclear Grade Nuclear Grade UO2 Powder ZrO2 Powder Blending Precompaction Coking Granulation UO Green 2 Final Compaction Pellets Chlorination Sintering Reduction Centreless Grinding Vacuum Distillation Sponge Handling Washing & Drying Zircaloy Stacking Ingots Compaction End Machining Alloying UO2 Green Appendage Welding Pellets EB Welding Graphite Coating -

US Nuclear Cooperation with India
U.S. Nuclear Cooperation with India: Issues for Congress Paul K. Kerr Analyst in Nonproliferation June 26, 2012 Congressional Research Service 7-5700 www.crs.gov RL33016 CRS Report for Congress Prepared for Members and Committees of Congress U.S. Nuclear Cooperation with India: Issues for Congress Summary India, which has not signed the Nuclear Nonproliferation Treaty and does not have International Atomic Energy Agency safeguards on all of its nuclear material, exploded a “peaceful” nuclear device in 1974, convincing the world of the need for greater restrictions on nuclear trade. The United States created the Nuclear Suppliers Group (NSG) as a direct response to India’s test, halted nuclear exports to India a few years later, and worked to convince other states to do the same. India tested nuclear weapons again in 1998. However, President Bush announced July 18, 2005, he would “work to achieve full civil nuclear energy cooperation with India” and would “also seek agreement from Congress to adjust U.S. laws and policies,” in the context of a broader partnership with India. U.S. nuclear cooperation with other countries is governed by the Atomic Energy Act (AEA) of 1954 (P.L. 95-242). However, P.L. 109-401, which President Bush signed into law on December 18, 2006, allows the President to waive several provisions of the AEA. On September 10, 2008, President Bush submitted to Congress, in addition to other required documents, a written determination that P.L. 109-401’s requirements for U.S. nuclear cooperation with India to proceed had been met. President Bush signed P.L. -

Industrial Visit to Nuclear Fuel Complex, Hyderabad on 27Th
INDUSTRIAL VISIT VISITED INDUSTRY:NFC(NUCLEAR FUEL COMPLEX) LOCATION OF THE INDUSTRY:NFC INDUSTRY, ECIL, Moula ali, Hyderabad, Telangana :500062 DATE OF INDUSTRY VISIT:13-02-2019 DEPARTMENT:CHEMICAL ENGINEERING YEAR:ENGINEERING 3rd YEAR CONTENTS: Introduction History Principle of production Flowchart How energy is generated Making of nuclear fuel Oath of thanks INTRODUCTION: The nuclear fuel complex(NFC) was established in 1971 as a major industrial unit of Indian’s department of atomic energy,for the supply of nuclear fuel bundles and reactor core components. It is a unique facility where natural and enriched fuel, zirconium alloy cladding and reactor core components are manufactured under one roof. HISTORY: NFC is a unit of department of atomic energy,Government of India. The complex is responsible for the supply of nuclear bundles and reactor core components for all the nuclear power reactors in India. It is a unique facility where the natural and enriched uranium fuel, zirconium alloy cladding and reactor core components are manufactured under one roof starting from the raw materials. PRINCIPLE OF PRODUCTION: As the power generated is low,then the per capita energy consumption is becoming low. There is insufficient power supply for all the people. Power generated Per capita energy consumption= Total population. since power generated by coal,thermal,tidal,solar energy is very less and it is inadequate for human usage. So by using nuclear energy they have been manufacturing uranium bundles which are used in generating electricity. PRODUCT: Uranium Bundles RAW MATERIALS: Magnesium di- uranate(MDU) Sand containing Zirconium FLOW CHART: FILTERATION DISSOLUTION SLURRY EXTRACTION PRECIPITATION COMPACTION STABILIZATION REDUCTION CALCINATION DRYING PELLET GRINDING PELLET READING SINTERING ENDCAP-WELDING NUCLEAR REACTOR INSPECTION END PLATE WELDING BUNDLE ASSEMBLY These are steps involved in the manufacturing of uranium rods which are used for the electricity generation. -
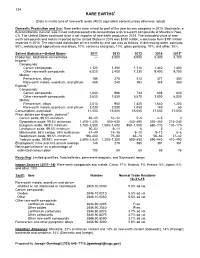
Mineral Commodity Summaries 2016
134 RARE EARTHS1 [Data in metric tons of rare-earth oxide (REO) equivalent content unless otherwise noted] Domestic Production and Use: Rare earths were mined for part of the year by one company in 2015. Bastnäsite, a fluorocarbonate mineral, was mined and processed into concentrates and rare-earth compounds at Mountain Pass, CA. The United States continued to be a net importer of rare-earth products in 2015. The estimated value of rare- earth compounds and metals imported by the United States in 2015 was $150 million, a decrease from $191 million imported in 2014. The estimated distribution of rare earths by end use was as follows, in decreasing order: catalysts, 60%; metallurgical applications and alloys, 10%; ceramics and glass, 10%; glass polishing, 10%; and other, 10%. Salient Statistics—United States: 2011 2012 2013 2014 2015e Production, bastnäsite concentrates — 3,000 5,500 5,400 4,100 Imports:2 Compounds: Cerium compounds 1,120 1,390 1,110 1,440 1,400 Other rare-earth compounds 6,020 3,400 7,330 9,400 9,700 Metals: Ferrocerium, alloys 186 276 313 371 360 Rare-earth metals, scandium, and yttrium 468 240 393 348 460 Exports:2 Compounds: Cerium compounds 1,640 996 734 608 600 Other rare-earth compounds 3,620 1,830 5,570 3,800 6,000 Metals: Ferrocerium, alloys 2,010 960 1,420 1,640 1,200 Rare-earth metals, scandium, and yttrium 3,030 2,080 1,050 140 60 Consumption, estimated 11,000 15,000 15,000 17,000 17,000 Price, dollars per kilogram, yearend:3 Cerium oxide, 99.5% minimum 40–45 10–12 5–6 4–5 2 Dysprosium oxide, 99.5% minimum 1,400–1,420 -

Governing Uranium in India
A Service of Leibniz-Informationszentrum econstor Wirtschaft Leibniz Information Centre Make Your Publications Visible. zbw for Economics Nayan, Rajiv Research Report Governing uranium in India DIIS Report, No. 2015:02 Provided in Cooperation with: Danish Institute for International Studies (DIIS), Copenhagen Suggested Citation: Nayan, Rajiv (2015) : Governing uranium in India, DIIS Report, No. 2015:02, ISBN 978-87-7605-736-7, Danish Institute for International Studies (DIIS), Copenhagen This Version is available at: http://hdl.handle.net/10419/120395 Standard-Nutzungsbedingungen: Terms of use: Die Dokumente auf EconStor dürfen zu eigenen wissenschaftlichen Documents in EconStor may be saved and copied for your Zwecken und zum Privatgebrauch gespeichert und kopiert werden. personal and scholarly purposes. Sie dürfen die Dokumente nicht für öffentliche oder kommerzielle You are not to copy documents for public or commercial Zwecke vervielfältigen, öffentlich ausstellen, öffentlich zugänglich purposes, to exhibit the documents publicly, to make them machen, vertreiben oder anderweitig nutzen. publicly available on the internet, or to distribute or otherwise use the documents in public. Sofern die Verfasser die Dokumente unter Open-Content-Lizenzen (insbesondere CC-Lizenzen) zur Verfügung gestellt haben sollten, If the documents have been made available under an Open gelten abweichend von diesen Nutzungsbedingungen die in der dort Content Licence (especially Creative Commons Licences), you genannten Lizenz gewährten Nutzungsrechte. may exercise -

Pre-Qualification Document
GOVERNMENT OF INDIA DEPARTMENT OF ATOMIC ENERGY NUCLEAR FUEL COMPLEX CIVIL ENGINEERING DIVISION HYDERABAD – 500 062 PH: 040-27184099/040-27184725 & Fax: 040-27122532 WORKS: CIVIL, PH AND STRUCTURAL STEEL AND OTHER ALLIED WORKS FOR ATMF PLANT AT NFC, HYD. PROJECT: ADVANCED TUBE MANUFACTURING FACILITY (ATMF) PQ NIT NOTICE NO.: C/ 1186/2018 PRE-QUALIFICATION DOCUMENT P a g e | 1 CONTENTS SECTION TITLE PAGE NIT Notice inviting tenders for Pre-qualification 4-22 Part : I Brief particulars of the work 23-24 Part : II Information & instructions for applicants 25-31 1. General 25 2. Definitions 26 3. Method of application 27 4. Final decision making Authority 27 5. Particulars provisional 27 6. Site visit 27 7. Initial criteria of eligibility 28 8. Evaluation criteria 29 9. Financial information 30 10.Experience in similar works 30 11.Organisation information 30 12.Construction plant & equipment 30 13. Preference for Green Building Norms 30 14.Letter of transmittal 30 15.Short listing the agencies 31 16.Award criteria 31 Part : III Information regarding eligibility 32-49 1. Letter of Transmittal 32-33 2. Form “A‟- Financial information 34 3. Form “B‟- Solvency certificate from Applicant’s Bankers 35 4.Form “C‟- Details of all works of similar class completed during 36- the last seven years. 37 5.Form “D‟- Projects under execution or awarded 38 6.Form “E‟- Performance report of works referred to in form “C” & 39 “D” 7.Form “F‟- Structure & organisation 40 8.Form “G‟- Details of technical & administrative personnel to be 41 employed for the work 9.Form “H‟- Details of construction plant & equipment likely to be 42- used in carrying out th e work 45 10.