PROTACTINIUM Element Symbol: Pa Atomic Number: 91
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table 2.Iii.1. Fissionable Isotopes1
FISSIONABLE ISOTOPES Charles P. Blair Last revised: 2012 “While several isotopes are theoretically fissionable, RANNSAD defines fissionable isotopes as either uranium-233 or 235; plutonium 238, 239, 240, 241, or 242, or Americium-241. See, Ackerman, Asal, Bale, Blair and Rethemeyer, Anatomizing Radiological and Nuclear Non-State Adversaries: Identifying the Adversary, p. 99-101, footnote #10, TABLE 2.III.1. FISSIONABLE ISOTOPES1 Isotope Availability Possible Fission Bare Critical Weapon-types mass2 Uranium-233 MEDIUM: DOE reportedly stores Gun-type or implosion-type 15 kg more than one metric ton of U- 233.3 Uranium-235 HIGH: As of 2007, 1700 metric Gun-type or implosion-type 50 kg tons of HEU existed globally, in both civilian and military stocks.4 Plutonium- HIGH: A separated global stock of Implosion 10 kg 238 plutonium, both civilian and military, of over 500 tons.5 Implosion 10 kg Plutonium- Produced in military and civilian 239 reactor fuels. Typically, reactor Plutonium- grade plutonium (RGP) consists Implosion 40 kg 240 of roughly 60 percent plutonium- Plutonium- 239, 25 percent plutonium-240, Implosion 10-13 kg nine percent plutonium-241, five 241 percent plutonium-242 and one Plutonium- percent plutonium-2386 (these Implosion 89 -100 kg 242 percentages are influenced by how long the fuel is irradiated in the reactor).7 1 This table is drawn, in part, from Charles P. Blair, “Jihadists and Nuclear Weapons,” in Gary A. Ackerman and Jeremy Tamsett, ed., Jihadists and Weapons of Mass Destruction: A Growing Threat (New York: Taylor and Francis, 2009), pp. 196-197. See also, David Albright N 2 “Bare critical mass” refers to the absence of an initiator or a reflector. -

An Introduction to Isotopic Calculations John M
An Introduction to Isotopic Calculations John M. Hayes ([email protected]) Woods Hole Oceanographic Institution, Woods Hole, MA 02543, USA, 30 September 2004 Abstract. These notes provide an introduction to: termed isotope effects. As a result of such effects, the • Methods for the expression of isotopic abundances, natural abundances of the stable isotopes of practically • Isotopic mass balances, and all elements involved in low-temperature geochemical • Isotope effects and their consequences in open and (< 200°C) and biological processes are not precisely con- closed systems. stant. Taking carbon as an example, the range of interest is roughly 0.00998 ≤ 13F ≤ 0.01121. Within that range, Notation. Absolute abundances of isotopes are com- differences as small as 0.00001 can provide information monly reported in terms of atom percent. For example, about the source of the carbon and about processes in 13 13 12 13 atom percent C = [ C/( C + C)]100 (1) which the carbon has participated. A closely related term is the fractional abundance The delta notation. Because the interesting isotopic 13 13 fractional abundance of C ≡ F differences between natural samples usually occur at and 13F = 13C/(12C + 13C) (2) beyond the third significant figure of the isotope ratio, it has become conventional to express isotopic abundances These variables deserve attention because they provide using a differential notation. To provide a concrete the only basis for perfectly accurate mass balances. example, it is far easier to say – and to remember – that Isotope ratios are also measures of the absolute abun- the isotope ratios of samples A and B differ by one part dance of isotopes; they are usually arranged so that the per thousand than to say that sample A has 0.3663 %15N more abundant isotope appears in the denominator and sample B has 0.3659 %15N. -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

FRANCIUM Element Symbol: Fr Atomic Number: 87
FRANCIUM Element Symbol: Fr Atomic Number: 87 An initiative of IYC 2011 brought to you by the RACI KAYE GREEN www.raci.org.au FRANCIUM Element symbol: Fr Atomic number: 87 Francium (previously known as eka-cesium and actinium K) is a radioactive metal and the second rarest naturally occurring element after Astatine. It is the least stable of the first 103 elements. Very little is known of the physical and chemical properties of Francium compared to other elements. Francium was discovered by Marguerite Perey of the Curie Institute in Paris, France in 1939. However, the existence of an element of atomic number 87 was predicted in the 1870s by Dmitri Mendeleev, creator of the first version of the periodic table, who presumed it would have chemical and physical properties similar to Cesium. Several research teams attempted to isolate this missing element, and there were at least four false claims of discovery during which it was named Russium (after the home country of soviet chemist D. K. Dobroserdov), Alkalinium (by English chemists Gerald J. K. Druce and Frederick H. Loring as the heaviest alkali metal), Virginium (after Virginia, home state of chemist Fred Allison), and Moldavium (by Horia Hulubei and Yvette Cauchois after Moldavia, the Romanian province where they conducted their work). Perey finally discovered Francium after purifying radioactive Actinium-227 from Lanthanum, and detecting particles decaying at low energy levels not previously identified. The new product exhibited chemical properties of an alkali metal (such as co-precipitating with Cesium salts), which led Perey to believe that it was element 87, caused by the alpha radioactive decay of Actinium-227. -

Toxicological Profile for Plutonium
PLUTONIUM 1 1. PUBLIC HEALTH STATEMENT This public health statement tells you about plutonium and the effects of exposure to it. The Environmental Protection Agency (EPA) identifies the most serious hazardous waste sites in the nation. These sites are then placed on the National Priorities List (NPL) and are targeted for long-term federal clean-up activities. Plutonium has been found in at least 16 of the 1,689 current or former NPL sites. Although the total number of NPL sites evaluated for this substance is not known, strict regulations make it unlikely that the number of sites at which plutonium is found would increase in the future as more sites are evaluated. This information is important because these sites may be sources of exposure and exposure to this substance may be harmful. When a substance is released from a large area, such as an industrial plant, or from a container, such as a drum or bottle, it enters the environment. This release does not always lead to exposure. You are normally exposed to a substance only when you come in contact with it. You may be exposed by breathing, eating, or drinking the substance, or by skin contact. However, since plutonium is radioactive, you can also be exposed to its radiation if you are near it. External exposure to radiation may occur from natural or man-made sources. Naturally occurring sources of radiation are cosmic radiation from space or radioactive materials in soil or building materials. Man- made sources of radioactive materials are found in consumer products, industrial equipment, atom bomb fallout, and to a smaller extent from hospital waste and nuclear reactors. -

The Separation of Bismuth-213 from Actinium-225 and the Ion Exchange Properties of the Alkali Metal Cations with an Inorganic Resin
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 12-2017 The Separation of Bismuth-213 from Actinium-225 and the Ion Exchange Properties of the Alkali Metal Cations with an Inorganic Resin Mark Alan Moore University of Tennessee Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Recommended Citation Moore, Mark Alan, "The Separation of Bismuth-213 from Actinium-225 and the Ion Exchange Properties of the Alkali Metal Cations with an Inorganic Resin. " PhD diss., University of Tennessee, 2017. https://trace.tennessee.edu/utk_graddiss/4848 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Mark Alan Moore entitled "The Separation of Bismuth-213 from Actinium-225 and the Ion Exchange Properties of the Alkali Metal Cations with an Inorganic Resin." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Chemical Engineering. Robert Counce, Major Professor We have read this dissertation and recommend its acceptance: Paul Dalhaimer, Howard Hall, George Schweitzer, Jack Watson Accepted for the Council: Dixie L. Thompson Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) The Separation of Bismuth-213 from Actinium-225 and the Ion Exchange Properties of the Alkali Metal Cations with an Inorganic Resin A Dissertation Presented for the Doctor of Philosophy Degree The University of Tennessee, Knoxville Mark Alan Moore December 2017 Copyright © 2017 by Mark A. -

Chromium-Nickel Steels Depleted of Nickel Stable Isotope Ni-58 As a Material for Fast Reactor Claddings
IAEA-CN-114/15p Chromium-Nickel steels depleted of nickel stable isotope Ni-58 as a material for fast reactor claddings G.L.Khorasanov, A.P.Ivanov, A.I.Blokhin, N.A.Demin Institute of Physics and Power Engineering named after A.I.Leypunsky 1, Bondarenko square, Obninsk, Kaluga region, 249033 Russia Tel.: +7-08439-98505, Fax: +7-095-2302326, E-mail: [email protected] INTRODUCTION Presently, the creation of new materials for fast reactor (FR) claddings is one of the main tasks of the nuclear engineering [1]. Now the Russian austenitic steel grade TchS68 with 15 % of nickel content is used as a material for the Russian FR BN-600 claddings [2]. With such a cladding the BN-600 fuel rod is stably operating during 10 years up to the fuel burn-up fraction of 10 % of heavy atoms (h.a.). The cladding material becomes swelled and unfit for further service after the fuel burn-up fraction of 10 % h.a. The task of increasing the fuel burn-up fraction up to 14 % h.a. is now the principal one for the BN-600 operation. For this purpose the new radiation–resistant austenitic steel grade EK164 with 19 % of nickel content is now developing in the Bochvar Institute, Russia [3]. It is generally assumed that steel swelling is caused by the combined action of radiation damage and helium accumulation in the irradiated material. In this case, nickel stable isotope, Ni-58 (its content in natural nickel is equal to 68%) is a source of helium creation. After 4 neutron capture via (n,g) reaction, Ni-58 is transmuted to radioisotope Ni-59 (T1/2=7.6⋅10 years) which has a high value of (n,α) reaction cross-section for thermal and epithermal neutrons. -

Lithium Isotope Fractionation During Uptake by Gibbsite
Available online at www.sciencedirect.com ScienceDirect Geochimica et Cosmochimica Acta 168 (2015) 133–150 www.elsevier.com/locate/gca Lithium isotope fractionation during uptake by gibbsite Josh Wimpenny a,⇑, Christopher A. Colla a, Ping Yu b, Qing-Zhu Yin a, James R. Rustad a, William H. Casey a,c a Department of Earth and Planetary Sciences, University of California, One Shields Avenue, Davis, CA 95616, United States b The Keck NMR Facility, University of California, One Shields Avenue, Davis, CA 95616, United States c Department of Chemistry, University of California, One Shields Avenue, Davis, CA 95616, United States Received 5 November 2014; accepted in revised form 8 July 2015; available online 15 July 2015 Abstract The intercalation of lithium from solution into the six-membered l2-oxo rings on the basal planes of gibbsite is well-constrained chemically. The product is a lithiated layered-double hydroxide solid that forms via in situ phase change. The reaction has well established kinetics and is associated with a distinct swelling of the gibbsite as counter ions enter the interlayer to balance the charge of lithiation. Lithium reacts to fill a fixed and well identifiable crystallographic site and has no solvation waters. Our lithium-isotope data shows that 6Li is favored during this intercalation and that the À À solid-solution fractionation depends on temperature, electrolyte concentration and counter ion identity (whether Cl ,NO3 À or ClO4 ). We find that the amount of isotopic fractionation between solid and solution (DLisolid-solution) varies with the amount of lithium taken up into the gibbsite structure, which itself depends upon the extent of conversion and also varies À À À with electrolyte concentration and in the counter ion in the order: ClO4 <NO3 <Cl . -

Isotopic Composition of Fission Gases in Lwr Fuel
XA0056233 ISOTOPIC COMPOSITION OF FISSION GASES IN LWR FUEL T. JONSSON Studsvik Nuclear AB, Hot Cell Laboratory, Nykoping, Sweden Abstract Many fuel rods from power reactors and test reactors have been punctured during past years for determination of fission gas release. In many cases the released gas was also analysed by mass spectrometry. The isotopic composition shows systematic variations between different rods, which are much larger than the uncertainties in the analysis. This paper discusses some possibilities and problems with use of the isotopic composition to decide from which part of the fuel the gas was released. In high burnup fuel from thermal reactors loaded with uranium fuel a significant part of the fissions occur in plutonium isotopes. The ratio Xe/Kr generated in the fuel is strongly dependent on the fissioning species. In addition, the isotopic composition of Kr and Xe shows a well detectable difference between fissions in different fissile nuclides. 1. INTRODUCTION Most LWRs use low enriched uranium oxide as fuel. Thermal fissions in U-235 dominate during the earlier part of the irradiation. Due to the build-up of heavier actinides during the irradiation fissions in Pu-239 and Pu-241 increase in importance as the burnup of the fuel increases. The composition of the fission products varies with the composition of the fuel and the irradiation conditions. The isotopic composition of fission gases is often determined in connection with measurement of gases in the plenum of punctured fuel rods. It can be of interest to discuss how more information on the fuel behaviour can be obtained by use of information available from already performed determinations of gas compositions. -
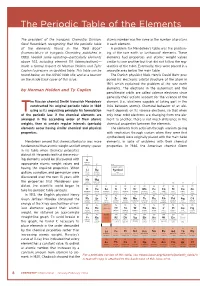
The Periodic Table of the Elements
The Periodic Table of the Elements The president of the Inorganic Chemistry Division, atomic number was the same as the number of protons Gerd Rosenblatt, recognizing that the periodic table in each element. of the elements found in the “Red Book” A problem for Mendeleev’s table was the position- (Nomenclature of Inorganic Chemistry, published in ing of the rare earth or lanthanoid* elements. These 1985) needed some updating—particularly elements elements had properties and atomic weight values above 103, including element 110 (darmstadtium)— similar to one another but that did not follow the reg- made a formal request to Norman Holden and Tyler ularities of the table. Eventually, they were placed in a Coplen to prepare an updated table. This table can be separate area below the main table. found below, on the IUPAC Web site, and as a tear-off The Danish physicist Niels Henrik David Bohr pro- on the inside back cover of this issue. posed his electronic orbital structure of the atom in 1921, which explained the problem of the rare earth by Norman Holden and Ty Coplen elements. The electrons in the outermost and the penultimate orbits are called valence electrons since generally their actions account for the valence of the he Russian chemist Dmitri Ivanovich Mendeleev element (i.e., electrons capable of taking part in the constructed his original periodic table in 1869 links between atoms). Chemical behavior of an ele- Tusing as its organizing principle his formulation ment depends on its valence electrons, so that when of the periodic law: if the chemical elements are only inner orbit electrons are changing from one ele- arranged in the ascending order of their atomic ment to another, there is not much difference in the weights, then at certain regular intervals (periods) chemical properties between the elements. -

Rapid Separations of Protactinium and Uranium Radioisotopes From
754 LETTERS TO THE EDITOR may be as small as one micron in diameter. Such particles are solutions with ether were required to further eliminate non products of breakdown or are introduced mto the enclosed system volatile foreign material which would extract into the ether. at the time of assembly. If special precautions were not taken to Ignition of the ether sample gave a weightless plate of the uranium eliminate stray particles, the subnormal breakdown strengths isotopes. obtained would lay within ±1O percent of a mean value which Approximate times required for the steps were: solution, for a given method of electrode polishing would be repeatable. extraction, and separation 10-20 seconds; washing 10-15 seconds; These mean values, reported earlier,! are in agreement with the and making of sample plate ("-'1 cc ether) ",15 seconds. results published by other workers. In nuclear chemical work where the main interest is in charac Experiments are continuing to determine whether the maximum terizing particular isotopes, emphasis is on radiochemical purity values reported here are indeed the intrinsic electric strengths of of the isotope rather than on chemical yield. The above procedures the liquids concerned. give only a 10-20 percent chemical yield-fa of which can be The author is indebted to the National Research Council of rapidly placed on a plate for counting, These yields are quite Canada for the award of a Fellowship which enabled him to carry satisfactory for the purpose, however, since contaminating isotopes out this work. have been reduced by a factor of 104 to lOS. Experimental results have shown that about 1 part in 104 of actinium, 1 part in lOS of 1 W. -

Periodic Table of the Elements Notes
Periodic Table of the Elements Notes Arrangement of the known elements based on atomic number and chemical and physical properties. Divided into three basic categories: Metals (left side of the table) Nonmetals (right side of the table) Metalloids (touching the zig zag line) Basic Organization by: Atomic structure Atomic number Chemical and Physical Properties Uses of the Periodic Table Useful in predicting: chemical behavior of the elements trends properties of the elements Atomic Structure Review: Atoms are made of protons, electrons, and neutrons. Elements are atoms of only one type. Elements are identified by the atomic number (# of protons in nucleus). Energy Levels Review: Electrons are arranged in a region around the nucleus called an electron cloud. Energy levels are located within the cloud. At least 1 energy level and as many as 7 energy levels exist in atoms Energy Levels & Valence Electrons Energy levels hold a specific amount of electrons: 1st level = up to 2 2nd level = up to 8 3rd level = up to 8 (first 18 elements only) The electrons in the outermost level are called valence electrons. Determine reactivity - how elements will react with others to form compounds Outermost level does not usually fill completely with electrons Using the Table to Identify Valence Electrons Elements are grouped into vertical columns because they have similar properties. These are called groups or families. Groups are numbered 1-18. Group numbers can help you determine the number of valence electrons: Group 1 has 1 valence electron. Group 2 has 2 valence electrons. Groups 3–12 are transition metals and have 1 or 2 valence electrons.