Garcinia Species in the Western Ghats: Phytochemical Perspective Chapter 7
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mundu:Garciniaxanthochymushookx Org. Dulcis(Roxb.) Kurz.?
Berita Biologi 9(6) - Desember 2009 MUNDU: Garciniaxanthochymus Hook.f. ATAU G. dulcis (Roxb.) Kurz.?1 [Mundu: Garciniaxanthochymus HookX or G. dulcis (Roxb.) Kurz.?] Nanda Utami2dan Rismita Sari3 2Herbarium Bogoriense, Bidang Botani, Pusat Penelitian Biologi-LIPI Cibinong Science Center, Jin. Raya Jakarta-Bogor, km 46, Cibinong 16911 e-mail: Utamil [email protected] 3Pusat Konservasi Tumbuhan Kebun Raya Indonesia, LIPI. Jl. Ir. H. Juanda 13, Bogor 16003 e-mail: [email protected] ABSTRACT "Mundu" is common name for one of the member of Clusiaceae family (manggis-manggisan). In scientific writing it is sometimes called Garcinia xanthochymus Hook.f. or G. dulcis (Roxb.) Kurz. To determine the correct name for these two kinds of plant a study was conducted to review its taxonomic status. Based on morphological data, anatomical and phylogenetic analysis it is showed that the two species is separated but there are closely related, and in according to International Code of Botanical Nomenclature Garcinia xanthochymus Hook.f is the correct name for "MUNDU" Kata kunci: Mundu, Clusiaceae, Garcinia xanthochymus Hook.f, G. dulcis (Roxb.) Kurz. PENDAHULUAN bunganya hampir tertutup, dengan diameter buah ± 2 Garcinia termasuk ke dalam suku/famili cm. Sedangkan Corner dan Watanabe (1969) manggis-manggisan, Guttiferae atau Clusiaceae, terdiri menyatukan kedua jenis tersebut tanpa ± 435 jenis, persebarannya dari Asia tenggara kemudian mengungkapkan alasan yang jelas. Berdasarkan meluas sampaiNew Caledonia, Australia utara, Afrika perbedaan pendapat ini, maka dalam makalah ini, tropik, Madagaskar, Polynesia, Central dan South dilakukan penelaahan kembali status taksonominya. Amerika Tengah dan Amerika Selatan (Jones, 1980). Diharapkan dari pengamatan ini dapat diketahui apakah Garcinia merupakan marga yang unik; tajuknya Mundu adalah G. -

Systematics and Floral Evolution in the Plant Genus Garcinia (Clusiaceae) Patrick Wayne Sweeney University of Missouri-St
University of Missouri, St. Louis IRL @ UMSL Dissertations UMSL Graduate Works 7-30-2008 Systematics and Floral Evolution in the Plant Genus Garcinia (Clusiaceae) Patrick Wayne Sweeney University of Missouri-St. Louis Follow this and additional works at: https://irl.umsl.edu/dissertation Part of the Biology Commons Recommended Citation Sweeney, Patrick Wayne, "Systematics and Floral Evolution in the Plant Genus Garcinia (Clusiaceae)" (2008). Dissertations. 539. https://irl.umsl.edu/dissertation/539 This Dissertation is brought to you for free and open access by the UMSL Graduate Works at IRL @ UMSL. It has been accepted for inclusion in Dissertations by an authorized administrator of IRL @ UMSL. For more information, please contact [email protected]. SYSTEMATICS AND FLORAL EVOLUTION IN THE PLANT GENUS GARCINIA (CLUSIACEAE) by PATRICK WAYNE SWEENEY M.S. Botany, University of Georgia, 1999 B.S. Biology, Georgia Southern University, 1994 A DISSERTATION Submitted to the Graduate School of the UNIVERSITY OF MISSOURI- ST. LOUIS In partial Fulfillment of the Requirements for the Degree DOCTOR OF PHILOSOPHY in BIOLOGY with an emphasis in Plant Systematics November, 2007 Advisory Committee Elizabeth A. Kellogg, Ph.D. Peter F. Stevens, Ph.D. P. Mick Richardson, Ph.D. Barbara A. Schaal, Ph.D. © Copyright 2007 by Patrick Wayne Sweeney All Rights Reserved Sweeney, Patrick, 2007, UMSL, p. 2 Dissertation Abstract The pantropical genus Garcinia (Clusiaceae), a group comprised of more than 250 species of dioecious trees and shrubs, is a common component of lowland tropical forests and is best known by the highly prized fruit of mangosteen (G. mangostana L.). The genus exhibits as extreme a diversity of floral form as is found anywhere in angiosperms and there are many unresolved taxonomic issues surrounding the genus. -

Garcinia Intermedia SCORE: -4.0 RATING: Low Risk (Pittier) Hammel
TAXON: Garcinia intermedia SCORE: -4.0 RATING: Low Risk (Pittier) Hammel Taxon: Garcinia intermedia (Pittier) Hammel Family: Clusiaceae Common Name(s): cherry mangosteen Synonym(s): Calophyllum edule Seem. lemon drop mangosteen Rheedia edulis (Seem.) Planch. & Triana monkey fruit Rheedia intermedia Pittier wildʹlemon rheedia Assessor: Chuck Chimera Status: Assessor Approved End Date: 1 Feb 2017 WRA Score: -4.0 Designation: L Rating: Low Risk Keywords: Tropical Tree, Edible Fruit, Shade-Tolerant, Dioecious, Animal-Dispersed Qsn # Question Answer Option Answer 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? 103 Does the species have weedy races? Species suited to tropical or subtropical climate(s) - If 201 island is primarily wet habitat, then substitute "wet (0-low; 1-intermediate; 2-high) (See Appendix 2) High tropical" for "tropical or subtropical" 202 Quality of climate match data (0-low; 1-intermediate; 2-high) (See Appendix 2) High 203 Broad climate suitability (environmental versatility) y=1, n=0 y Native or naturalized in regions with tropical or 204 y=1, n=0 y subtropical climates Does the species have a history of repeated introductions 205 y=-2, ?=-1, n=0 y outside its natural range? 301 Naturalized beyond native range y = 1*multiplier (see Appendix 2), n= question 205 n 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see Appendix 2) n 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see Appendix 2) n 304 Environmental weed n=0, y = 2*multiplier -

Floristic and Phytoclimatic Study of an Indigenous Small Scale Natural Landscape Vegetation of Jhargram District, West Bengal, India
DOI: https://doi.org/10.31357/jtfe.v10i1.4686 Sen and Bhakat/ Journal of Tropical Forestry and Environment Vol. 10 No. 01 (2020) 17-39 Floristic and Phytoclimatic Study of an Indigenous Small Scale Natural Landscape Vegetation of Jhargram District, West Bengal, India U.K. Sen* and R.K. Bhakat Ecology and Taxonomy Laboratory, Department of Botany and Forestry, Vidyasagar University, West Bengal, India Date Received: 29-09-2019 Date Accepted: 28-06-2020 Abstract Sacred groves are distinctive examples of biotic components as genetic resources being preserved in situ and serve as secure heavens for many endangered and endemic taxa. From this point of view, the biological spectrum, leaf spectrum and conservation status of the current sacred grove vegetation, SBT (Swarga Bauri Than) in Jhargram district of West Bengal, India, have been studied. The area's floristic study revealed that SBT‟s angiosperms were varied and consisted of 307 species belonging to 249 genera, distributed under 79 families of 36 orders as per APG IV. Fabales (12.05%) and Fabaceae (11.73%) are the dominant order and family in terms of species wealth. Biological spectrum indicates that the region enjoys “thero-chamae-cryptophytic” type of phytoclimate. With respect to the spectrum of the leaf size, mesophyll (14.05%) was found to be high followed by notophyll (7.84%), microphyll (7.19%), macrophyll (7.84%), nanophyll (6.86%), leptophyll (6.21%), and megaphyll (2.29%). The study area, being a sacred grove, it has a comparatively undisturbed status, and the protection of germplasm in the grove is based on traditional belief in the social system. -

Systematics and Floral Evolution in the Plant Genus Garcinia (Clusiaceae)
University of Missouri, St. Louis IRL @ UMSL Dissertations UMSL Graduate Works 7-30-2008 Systematics and Floral Evolution in the Plant Genus Garcinia (Clusiaceae) Patrick Wayne Sweeney University of Missouri-St. Louis Follow this and additional works at: https://irl.umsl.edu/dissertation Part of the Biology Commons Recommended Citation Sweeney, Patrick Wayne, "Systematics and Floral Evolution in the Plant Genus Garcinia (Clusiaceae)" (2008). Dissertations. 539. https://irl.umsl.edu/dissertation/539 This Dissertation is brought to you for free and open access by the UMSL Graduate Works at IRL @ UMSL. It has been accepted for inclusion in Dissertations by an authorized administrator of IRL @ UMSL. For more information, please contact [email protected]. SYSTEMATICS AND FLORAL EVOLUTION IN THE PLANT GENUS GARCINIA (CLUSIACEAE) by PATRICK WAYNE SWEENEY M.S. Botany, University of Georgia, 1999 B.S. Biology, Georgia Southern University, 1994 A DISSERTATION Submitted to the Graduate School of the UNIVERSITY OF MISSOURI- ST. LOUIS In partial Fulfillment of the Requirements for the Degree DOCTOR OF PHILOSOPHY in BIOLOGY with an emphasis in Plant Systematics November, 2007 Advisory Committee Elizabeth A. Kellogg, Ph.D. Peter F. Stevens, Ph.D. P. Mick Richardson, Ph.D. Barbara A. Schaal, Ph.D. © Copyright 2007 by Patrick Wayne Sweeney All Rights Reserved Sweeney, Patrick, 2007, UMSL, p. 2 Dissertation Abstract The pantropical genus Garcinia (Clusiaceae), a group comprised of more than 250 species of dioecious trees and shrubs, is a common component of lowland tropical forests and is best known by the highly prized fruit of mangosteen (G. mangostana L.). The genus exhibits as extreme a diversity of floral form as is found anywhere in angiosperms and there are many unresolved taxonomic issues surrounding the genus. -

Gogoi P, Nath N. Diversity and Inventorization of Angiospermic Flora in Dibrugarh District, Assam, Northeast India. Plant Science Today
1 Gogoi P, Nath N. Diversity and inventorization of angiospermic flora in Dibrugarh district, Assam, Northeast India. Plant Science Today. 2021;8(3):621–628. https://doi.org/10.14719/pst.2021.8.3.1118 Supplementary Tables Table 1. Angiosperm Phylogeny Group (APG IV) Classification of angiosperm taxa from Dibrugarh District. Families according to B&H Superorder/Order Family and Species System along with family Common name Habit Nativity Uses number BASAL ANGIOSPERMS APG IV Nymphaeales Nymphaeaceae Nymphaea nouchali 8.Nymphaeaceae Boga-bhet Aquatic Herb Native Edible Burm.f. Nymphaea rubra Roxb. Mokua/ Ronga 8.Nymphaeaceae Aquatic Herb Native Medicinal ex Andrews bhet MAGNOLIIDS Piperales Saururaceae Houttuynia cordata 139.(A) Mosondori Herb Native Medicinal Thunb. Saururaceae Piperaceae Piper longum L. 139.Piperaceae Bon Jaluk Climber Native Medicinal Piper nigrum L. 139.Piperaceae Jaluk Climber Native Medicinal Piper thomsonii (C.DC.) 139.Piperaceae Aoni pan Climber Native Medicinal Hook.f. Peperomia mexicana Invasive/ 139.Piperaceae Pithgoch Herb (Miq.) Miq. SAM Aristolochiaceae Aristolochia ringens Invasive/ 138.Aristolochiaceae Arkomul Climber Medicinal Vahl TAM Magnoliales Magnolia griffithii 4.Magnoliaceae Gahori-sopa Tree Native Wood Hook.f. & Thomson Magnolia hodgsonii (Hook.f. & Thomson) 4.Magnoliaceae Borhomthuri Tree Native Cosmetic H.Keng Magnolia insignis Wall. 4.Magnoliaceae Phul sopa Tree Native Magnolia champaca (L.) 4.Magnoliaceae Tita-sopa Tree Native Medicinal Baill. ex Pierre Magnolia mannii (King) Figlar 4.Magnoliaceae Kotholua-sopa Tree Native Annonaceae Annona reticulata L. 5.Annonaceae Atlas Tree Native Edible Annona squamosa L. 5.Annonaceae Atlas Tree Invasive/WI Edible Monoon longifolium Medicinal/ (Sonn.) B. Xue & R.M.S. 5.Annonaceae Debodaru Tree Exotic/SR Biofencing Saunders Laurales Lauraceae Actinodaphne obovata 143.Lauraceae Noga-baghnola Tree Native (Nees) Blume Beilschmiedia assamica 143.Lauraceae Kothal-patia Tree Native Meisn. -

Structural Diversity of Secondary Metabolites in Garcinia Species
JNTBGRI Diversity of Garcinia species in the Western Ghats: Phytochemical Perspective Chapter 2 Structural diversity of secondary metabolites in Garcinia species A. P. Anu Aravind, Lekshmi N. Menon and K. B. Rameshkumar* Phytochemistry and Phytopharmacology Division Jawaharlal Nehru Tropical Botanic Garden and Research Institute Palode, Thiruvananthapuram-695562, Kerala, India * Corresponding author Abstract Plants of the genus Garcinia produce structurally diverse secondary metabolites such as biflavonoids, xanthones, benzophenones, flavonoids, biphenyls, acyl phloroglucinols, depsidones and terpenoids. The rich diversity in chemical structures made the genus Garcinia attractive for the phytochemists. In addition, several industrial sectors such as cosmetic, food, pharmaceutics, neutraceutics and paints are centered around the genus. The genus is represented by more than 250 species, among which nearly 120 species were subjected to phytochemical investigation. A review of the structural diversity of secondary metabolites of Garcinia species revealed that xanthones are the important class of secondary metabolites, distributed in 74 Garcinia species, followed by benzophenones in 50 species and biflavonoids in 45 species. Biphenyls, acyl phloroglucinols, depsidones and flavonoids are some other interesting group of phenolic compounds in Garcinia species. The present chapter enlists the major phenolic compounds reported from Garcinia species. Keywords: Garcinia, Secondary metabolites, Xanthones, Biflavonoids, Benzophenones Introduction Plants -

Diversity of Garcinia Species in the Western Ghats: Phytochemical Perspective
Diversity of Garcinia species in the Western Ghats: Phytochemical Perspective Editor K. B. Rameshkumar Jawaharlal Nehru Tropical Botanic Garden and Research Institute Thiruvananthapuram Title: Diversity of Garcinia species in the Western Ghats: Phytochemical Perspective Editor: K. B. Rameshkumar Published by: Jawaharlal Nehru Tropical Botanic Garden and Research Institute, Palode, Thiruvananthapuram 695 562, Kerala, India ISBN No.: 978-81-924674-5-0 Printed at: Akshara Offset, Thiruvananthapuram- 695 035 Copyright © 2016: Editor and Publisher All rights reserved. This book may not be reproduced in whole or in part without the prior written permission of the copyright owner. ii Foreword I am delighted to write a Foreword to the Book ‘Diversity of Garcinia species in the Western Ghats: Phytochemical Perspective’ edited by my student Dr. K. B. Rameshkumar who took Garcinia imberti as a subject for his doctoral studies. It gives me all the more pleasure and gratification to see that he continued with his studies on Garcinia species of the Western Ghats along with his students and colleagues. Unlike many other doctoral students, he kept alive his passion for the studies on Garcinia and the present book is the outcome of his dedicated efforts during the last one and a half decades. Pursuit of science is a passion and unravelling the subtleties of nature is an ecstasy which fulfils the inner urge for quest and discovery. The genus Garcinia is important by virtue of their reputation in traditional medicines, established pharmacological activities, diversity in chemical structures and potential nutritional properties. Despite recent progress in phytochemical and pharmacological studies on Garcinia species world over, significant gaps still exist concerning the exploration of the vast data on phytochemical diversity of Garcinia species. -

Andaman & Nicobar Islands, India
RESEARCH Vol. 21, Issue 68, 2020 RESEARCH ARTICLE ISSN 2319–5746 EISSN 2319–5754 Species Floristic Diversity and Analysis of South Andaman Islands (South Andaman District), Andaman & Nicobar Islands, India Mudavath Chennakesavulu Naik1, Lal Ji Singh1, Ganeshaiah KN2 1Botanical Survey of India, Andaman & Nicobar Regional Centre, Port Blair-744102, Andaman & Nicobar Islands, India 2Dept of Forestry and Environmental Sciences, School of Ecology and Conservation, G.K.V.K, UASB, Bangalore-560065, India Corresponding author: Botanical Survey of India, Andaman & Nicobar Regional Centre, Port Blair-744102, Andaman & Nicobar Islands, India Email: [email protected] Article History Received: 01 October 2020 Accepted: 17 November 2020 Published: November 2020 Citation Mudavath Chennakesavulu Naik, Lal Ji Singh, Ganeshaiah KN. Floristic Diversity and Analysis of South Andaman Islands (South Andaman District), Andaman & Nicobar Islands, India. Species, 2020, 21(68), 343-409 Publication License This work is licensed under a Creative Commons Attribution 4.0 International License. General Note Article is recommended to print as color digital version in recycled paper. ABSTRACT After 7 years of intensive explorations during 2013-2020 in South Andaman Islands, we recorded a total of 1376 wild and naturalized vascular plant taxa representing 1364 species belonging to 701 genera and 153 families, of which 95% of the taxa are based on primary collections. Of the 319 endemic species of Andaman and Nicobar Islands, 111 species are located in South Andaman Islands and 35 of them strict endemics to this region. 343 Page Key words: Vascular Plant Diversity, Floristic Analysis, Endemcity. © 2020 Discovery Publication. All Rights Reserved. www.discoveryjournals.org OPEN ACCESS RESEARCH ARTICLE 1. -
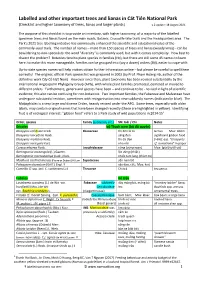
Labelled and Other Important Trees and Lianas in Cát Tiên National Park (Checklist and Higher Taxonomy of Trees, Lianas and Larger Plants) V.2 Update: 18 August 2021
Labelled and other important trees and lianas in Cát Tiên National Park (Checklist and higher taxonomy of trees, lianas and larger plants) v.2 update: 18 August 2021 The purpose of this checklist is to provide an inventory, with higher taxonomy, of a majority of the labelled specimen trees and lianas found on the main roads, Botanic, Crocodile-lake trails and the Headquarters area. The Park's 2021 tree labelling initiative has enormously enhanced the scientific and educational value of the commonly-used trails. The number of names – more than 150 species of trees and lianas (woody vines) - can be bewildering to non-specialists: the word "diversity" is commonly used, but with it comes complexity. How best to dissect the problem? Botanists tend to place species in families (Họ), but there are still some 45 names to learn here; to make this more manageable, families can be grouped into (say a dozen) orders (Bộ), easier to cope-with. Up-to-date species names will help visitors obtain further information online – but please be careful to spell them correctly! The original, official Park species list was prepared in 2002 (by Prof. Phạm Hoàng Hộ, author of the definitive work Cây Cỏ Việt Nam). However since then, plant taxonomy has been revised substantially by the international Angiosperm Phylogeny Group (APG), with whole plant families promoted, demoted or moved to different orders. Furthermore, genera and species have been – and continue to be - revised in light of scientific evidence; this also can be confusing for non-botanists. Two important families, the Fabaceae and Malvaceae have undergone substantial revision, sometimes with reorganisation into new subfamily names (indicated in blue). -

Phylogenetic Distribution and Evolution of Mycorrhizas in Land Plants
Mycorrhiza (2006) 16: 299–363 DOI 10.1007/s00572-005-0033-6 REVIEW B. Wang . Y.-L. Qiu Phylogenetic distribution and evolution of mycorrhizas in land plants Received: 22 June 2005 / Accepted: 15 December 2005 / Published online: 6 May 2006 # Springer-Verlag 2006 Abstract A survey of 659 papers mostly published since plants (Pirozynski and Malloch 1975; Malloch et al. 1980; 1987 was conducted to compile a checklist of mycorrhizal Harley and Harley 1987; Trappe 1987; Selosse and Le Tacon occurrence among 3,617 species (263 families) of land 1998;Readetal.2000; Brundrett 2002). Since Nägeli first plants. A plant phylogeny was then used to map the my- described them in 1842 (see Koide and Mosse 2004), only a corrhizal information to examine evolutionary patterns. Sev- few major surveys have been conducted on their phyloge- eral findings from this survey enhance our understanding of netic distribution in various groups of land plants either by the roles of mycorrhizas in the origin and subsequent diver- retrieving information from literature or through direct ob- sification of land plants. First, 80 and 92% of surveyed land servation (Trappe 1987; Harley and Harley 1987;Newman plant species and families are mycorrhizal. Second, arbus- and Reddell 1987). Trappe (1987) gathered information on cular mycorrhiza (AM) is the predominant and ancestral type the presence and absence of mycorrhizas in 6,507 species of of mycorrhiza in land plants. Its occurrence in a vast majority angiosperms investigated in previous studies and mapped the of land plants and early-diverging lineages of liverworts phylogenetic distribution of mycorrhizas using the classifi- suggests that the origin of AM probably coincided with the cation system by Cronquist (1981). -

Clusiaceae.Pdf
CLUSIACEAE (GUTTIFERAE) 藤黄科 teng huang ke Li Xiwen (李锡文 Li Hsi-wen)1, Li Jie (李捷)2; Norman K. B. Robson3, Peter F. Stevens4 Trees, shrubs, or sometimes herbs containing resin or oil in schizogenous spaces or canals and sometimes black or red glands containing hypericin or pseudohypericin. Leaves simple, entire or rarely gland-fringed, opposite or sometimes whorled, nearly always estipulate. Flowers bisexual or unisexual, regular, hypogynous, solitary or in cymes or thyrses; bracteoles often inserted just beneath calyx and then not always easily distinguishable from sepals. Sepals (2–)4 or 5(or 6), imbricate or decussate or rarely wholly united in bud, inner ones sometimes petaloid. Petals [3 or]4 or 5[or 6], free, imbricate or contorted in bud. Stamens many to rarely few (9), in [3 or]4 or 5 bundles (fascicles) that are free and antipetalous or variously connate, with filaments variously united or apparently free and then sometimes sterile (staminodes); anther dehiscence longitudinal. Staminode bundles (fasciclodes) 3–5, free and antisepalous or variously connate or absent. Ovary superior, with 2–5(–12) connate carpels, 1–12-loculed, with axile to parietal or basal placentation; ovules 1 to many on each placenta, erect to pendulous; styles 1–5[–12], free or ± united or absent; stigmas 1– 12, punctiform to peltate or, when sessile, radiate, surface papillate or smooth. Fruit a septicidal or septifragal, rarely loculicidal, capsule, berry, or drupe; seeds 1 to many, without or almost without endosperm [sometimes arillate]. About 40 genera and 1200 species: mainly in tropical regions, except Hypericum and Triadenum, which are both mainly temperate in distribu- tion; eight genera (one endemic) and 95 species (48 endemic, one introduced) in China.