Count Data Analysis in Randomised Clinical Trials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Introduction to Poisson Regression Russ Lavery, K&L Consulting Services, King of Prussia, PA, U.S.A
NESUG 2010 Statistics and Analysis An Animated Guide: An Introduction To Poisson Regression Russ Lavery, K&L Consulting Services, King of Prussia, PA, U.S.A. ABSTRACT: This paper will be a brief introduction to Poisson regression (theory, steps to be followed, complications and interpretation) via a worked example. It is hoped that this will increase motivation towards learning this useful statistical technique. INTRODUCTION: Poisson regression is available in SAS through the GENMOD procedure (generalized modeling). It is appropriate when: 1) the process that generates the conditional Y distributions would, theoretically, be expected to be a Poisson random process and 2) when there is no evidence of overdispersion and 3) when the mean of the marginal distribution is less than ten (preferably less than five and ideally close to one). THE POISSON DISTRIBUTION: The Poison distribution is a discrete Percent of observations where the random variable X is expected distribution and is appropriate for to have the value x, given that the Poisson distribution has a mean modeling counts of observations. of λ= P(X=x, λ ) = (e - λ * λ X) / X! Counts are observed cases, like the 0.4 count of measles cases in cities. You λ can simply model counts if all data 0.35 were collected in the same measuring 0.3 unit (e.g. the same number of days or 0.3 0.5 0.8 same number of square feet). 0.25 λ 1 0.2 3 You can use the Poisson Distribution = 5 for modeling rates (rates are counts 0.15 20 per unit) if the units of collection were 8 different. -

Generalized Linear Models (Glms)
San Jos´eState University Math 261A: Regression Theory & Methods Generalized Linear Models (GLMs) Dr. Guangliang Chen This lecture is based on the following textbook sections: • Chapter 13: 13.1 – 13.3 Outline of this presentation: • What is a GLM? • Logistic regression • Poisson regression Generalized Linear Models (GLMs) What is a GLM? In ordinary linear regression, we assume that the response is a linear function of the regressors plus Gaussian noise: 0 2 y = β0 + β1x1 + ··· + βkxk + ∼ N(x β, σ ) | {z } |{z} linear form x0β N(0,σ2) noise The model can be reformulate in terms of • distribution of the response: y | x ∼ N(µ, σ2), and • dependence of the mean on the predictors: µ = E(y | x) = x0β Dr. Guangliang Chen | Mathematics & Statistics, San Jos´e State University3/24 Generalized Linear Models (GLMs) beta=(1,2) 5 4 3 β0 + β1x b y 2 y 1 0 −1 0.0 0.2 0.4 0.6 0.8 1.0 x x Dr. Guangliang Chen | Mathematics & Statistics, San Jos´e State University4/24 Generalized Linear Models (GLMs) Generalized linear models (GLM) extend linear regression by allowing the response variable to have • a general distribution (with mean µ = E(y | x)) and • a mean that depends on the predictors through a link function g: That is, g(µ) = β0x or equivalently, µ = g−1(β0x) Dr. Guangliang Chen | Mathematics & Statistics, San Jos´e State University5/24 Generalized Linear Models (GLMs) In GLM, the response is typically assumed to have a distribution in the exponential family, which is a large class of probability distributions that have pdfs of the form f(x | θ) = a(x)b(θ) exp(c(θ) · T (x)), including • Normal - ordinary linear regression • Bernoulli - Logistic regression, modeling binary data • Binomial - Multinomial logistic regression, modeling general cate- gorical data • Poisson - Poisson regression, modeling count data • Exponential, Gamma - survival analysis Dr. -

A Simple Censored Median Regression Estimator
Statistica Sinica 16(2006), 1043-1058 A SIMPLE CENSORED MEDIAN REGRESSION ESTIMATOR Lingzhi Zhou The Hong Kong University of Science and Technology Abstract: Ying, Jung and Wei (1995) proposed an estimation procedure for the censored median regression model that regresses the median of the survival time, or its transform, on the covariates. The procedure requires solving complicated nonlinear equations and thus can be very difficult to implement in practice, es- pecially when there are multiple covariates. Moreover, the asymptotic covariance matrix of the estimator involves the density of the errors that cannot be esti- mated reliably. In this paper, we propose a new estimator for the censored median regression model. Our estimation procedure involves solving some convex min- imization problems and can be easily implemented through linear programming (Koenker and D'Orey (1987)). In addition, a resampling method is presented for estimating the covariance matrix of the new estimator. Numerical studies indi- cate the superiority of the finite sample performance of our estimator over that in Ying, Jung and Wei (1995). Key words and phrases: Censoring, convexity, LAD, resampling. 1. Introduction The accelerated failure time (AFT) model, which relates the logarithm of the survival time to covariates, is an attractive alternative to the popular Cox (1972) proportional hazards model due to its ease of interpretation. The model assumes that the failure time T , or some monotonic transformation of it, is linearly related to the covariate vector Z 0 Ti = β0Zi + "i; i = 1; : : : ; n: (1.1) Under censoring, we only observe Yi = min(Ti; Ci), where Ci are censoring times, and Ti and Ci are independent conditional on Zi. -

Cluster Analysis, a Powerful Tool for Data Analysis in Education
International Statistical Institute, 56th Session, 2007: Rita Vasconcelos, Mßrcia Baptista Cluster Analysis, a powerful tool for data analysis in Education Vasconcelos, Rita Universidade da Madeira, Department of Mathematics and Engeneering Caminho da Penteada 9000-390 Funchal, Portugal E-mail: [email protected] Baptista, Márcia Direcção Regional de Saúde Pública Rua das Pretas 9000 Funchal, Portugal E-mail: [email protected] 1. Introduction A database was created after an inquiry to 14-15 - year old students, which was developed with the purpose of identifying the factors that could socially and pedagogically frame the results in Mathematics. The data was collected in eight schools in Funchal (Madeira Island), and we performed a Cluster Analysis as a first multivariate statistical approach to this database. We also developed a logistic regression analysis, as the study was carried out as a contribution to explain the success/failure in Mathematics. As a final step, the responses of both statistical analysis were studied. 2. Cluster Analysis approach The questions that arise when we try to frame socially and pedagogically the results in Mathematics of 14-15 - year old students, are concerned with the types of decisive factors in those results. It is somehow underlying our objectives to classify the students according to the factors understood by us as being decisive in students’ results. This is exactly the aim of Cluster Analysis. The hierarchical solution that can be observed in the dendogram presented in the next page, suggests that we should consider the 3 following clusters, since the distances increase substantially after it: Variables in Cluster1: mother qualifications; father qualifications; student’s results in Mathematics as classified by the school teacher; student’s results in the exam of Mathematics; time spent studying. -

Generalized Linear Models with Poisson Family: Applications in Ecology
UNIVERSITY OF ABOMEY- CALAVI *********** FACULTY OF AGRONOMIC SCIENCES *************** **************** Master Program in Statistics, Major Biostatistics 1st batch Generalized linear models with Poisson family: applications in ecology A thesis submitted to the Faculty of Agronomic Sciences in partial fulfillment of the requirements for the degree of the Master of Sciences in Biostatistics Presented by: LOKONON Enagnon Bruno Supervisor: Pr Romain L. GLELE KAKAÏ, Professor of Biostatistics and Forest estimation Academic year: 2014-2015 UNIVERSITE D’ABOMEY- CALAVI *********** FACULTE DES SCIENCES AGRONOMIQUES *************** ************** Programme de Master en Biostatistiques 1ère Promotion Modèles linéaires généralisés de la famille de Poisson : applications en écologie Mémoire soumis à la Faculté des Sciences Agronomiques pour obtenir le Diplôme de Master recherche en Biostatistiques Présenté par: LOKONON Enagnon Bruno Superviseur: Pr Romain L. GLELE KAKAÏ, Professeur titulaire de Biostatistiques et estimation forestière Année académique: 2014-2015 Certification I certify that this work has been achieved by LOKONON E. Bruno under my entire supervision at the University of Abomey-Calavi (Benin) in order to obtain his Master of Science degree in Biostatistics. Pr Romain L. GLELE KAKAÏ Professor of Biostatistics and Forest estimation i Acknowledgements This research was supported by WAAPP/PPAAO-BENIN (West African Agricultural Productivity Program/ Programme de Productivité Agricole en Afrique de l‟Ouest). This dissertation could only have been possible through the generous contributions of many people. First and foremost, I am grateful to my supervisor Pr Romain L. GLELE KAKAÏ, Professor of Biostatistics and Forest estimation who tirelessly played key role in orientation, scientific writing and mentoring during this research. In particular, I thank him for his prompt availability whenever needed. -

The Detection of Heteroscedasticity in Regression Models for Psychological Data
Psychological Test and Assessment Modeling, Volume 58, 2016 (4), 567-592 The detection of heteroscedasticity in regression models for psychological data Andreas G. Klein1, Carla Gerhard2, Rebecca D. Büchner2, Stefan Diestel3 & Karin Schermelleh-Engel2 Abstract One assumption of multiple regression analysis is homoscedasticity of errors. Heteroscedasticity, as often found in psychological or behavioral data, may result from misspecification due to overlooked nonlinear predictor terms or to unobserved predictors not included in the model. Although methods exist to test for heteroscedasticity, they require a parametric model for specifying the structure of heteroscedasticity. The aim of this article is to propose a simple measure of heteroscedasticity, which does not need a parametric model and is able to detect omitted nonlinear terms. This measure utilizes the dispersion of the squared regression residuals. Simulation studies show that the measure performs satisfactorily with regard to Type I error rates and power when sample size and effect size are large enough. It outperforms the Breusch-Pagan test when a nonlinear term is omitted in the analysis model. We also demonstrate the performance of the measure using a data set from industrial psychology. Keywords: Heteroscedasticity, Monte Carlo study, regression, interaction effect, quadratic effect 1Correspondence concerning this article should be addressed to: Prof. Dr. Andreas G. Klein, Department of Psychology, Goethe University Frankfurt, Theodor-W.-Adorno-Platz 6, 60629 Frankfurt; email: [email protected] 2Goethe University Frankfurt 3International School of Management Dortmund Leipniz-Research Centre for Working Environment and Human Factors 568 A. G. Klein, C. Gerhard, R. D. Büchner, S. Diestel & K. Schermelleh-Engel Introduction One of the standard assumptions underlying a linear model is that the errors are inde- pendently identically distributed (i.i.d.). -
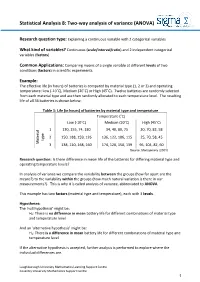
Statistical Analysis 8: Two-Way Analysis of Variance (ANOVA)
Statistical Analysis 8: Two-way analysis of variance (ANOVA) Research question type: Explaining a continuous variable with 2 categorical variables What kind of variables? Continuous (scale/interval/ratio) and 2 independent categorical variables (factors) Common Applications: Comparing means of a single variable at different levels of two conditions (factors) in scientific experiments. Example: The effective life (in hours) of batteries is compared by material type (1, 2 or 3) and operating temperature: Low (-10˚C), Medium (20˚C) or High (45˚C). Twelve batteries are randomly selected from each material type and are then randomly allocated to each temperature level. The resulting life of all 36 batteries is shown below: Table 1: Life (in hours) of batteries by material type and temperature Temperature (˚C) Low (-10˚C) Medium (20˚C) High (45˚C) 1 130, 155, 74, 180 34, 40, 80, 75 20, 70, 82, 58 2 150, 188, 159, 126 136, 122, 106, 115 25, 70, 58, 45 type Material 3 138, 110, 168, 160 174, 120, 150, 139 96, 104, 82, 60 Source: Montgomery (2001) Research question: Is there difference in mean life of the batteries for differing material type and operating temperature levels? In analysis of variance we compare the variability between the groups (how far apart are the means?) to the variability within the groups (how much natural variation is there in our measurements?). This is why it is called analysis of variance, abbreviated to ANOVA. This example has two factors (material type and temperature), each with 3 levels. Hypotheses: The 'null hypothesis' might be: H0: There is no difference in mean battery life for different combinations of material type and temperature level And an 'alternative hypothesis' might be: H1: There is a difference in mean battery life for different combinations of material type and temperature level If the alternative hypothesis is accepted, further analysis is performed to explore where the individual differences are. -

Testing Hypotheses for Differences Between Linear Regression Lines
United States Department of Testing Hypotheses for Differences Agriculture Between Linear Regression Lines Forest Service Stanley J. Zarnoch Southern Research Station e-Research Note SRS–17 May 2009 Abstract The general linear model (GLM) is often used to test for differences between means, as for example when the Five hypotheses are identified for testing differences between simple linear analysis of variance is used in data analysis for designed regression lines. The distinctions between these hypotheses are based on a priori assumptions and illustrated with full and reduced models. The studies. However, the GLM has a much wider application contrast approach is presented as an easy and complete method for testing and can also be used to compare regression lines. The for overall differences between the regressions and for making pairwise GLM allows for the use of both qualitative and quantitative comparisons. Use of the Bonferroni adjustment to ensure a desired predictor variables. Quantitative variables are continuous experimentwise type I error rate is emphasized. SAS software is used to illustrate application of these concepts to an artificial simulated dataset. The values such as diameter at breast height, tree height, SAS code is provided for each of the five hypotheses and for the contrasts age, or temperature. However, many predictor variables for the general test and all possible specific tests. in the natural resources field are qualitative. Examples of qualitative variables include tree class (dominant, Keywords: Contrasts, dummy variables, F-test, intercept, SAS, slope, test of conditional error. codominant); species (loblolly, slash, longleaf); and cover type (clearcut, shelterwood, seed tree). Fraedrich and others (1994) compared linear regressions of slash pine cone specific gravity and moisture content Introduction on collection date between families of slash pine grown in a seed orchard. -

5. Dummy-Variable Regression and Analysis of Variance
Sociology 740 John Fox Lecture Notes 5. Dummy-Variable Regression and Analysis of Variance Copyright © 2014 by John Fox Dummy-Variable Regression and Analysis of Variance 1 1. Introduction I One of the limitations of multiple-regression analysis is that it accommo- dates only quantitative explanatory variables. I Dummy-variable regressors can be used to incorporate qualitative explanatory variables into a linear model, substantially expanding the range of application of regression analysis. c 2014 by John Fox Sociology 740 ° Dummy-Variable Regression and Analysis of Variance 2 2. Goals: I To show how dummy regessors can be used to represent the categories of a qualitative explanatory variable in a regression model. I To introduce the concept of interaction between explanatory variables, and to show how interactions can be incorporated into a regression model by forming interaction regressors. I To introduce the principle of marginality, which serves as a guide to constructing and testing terms in complex linear models. I To show how incremental I -testsareemployedtotesttermsindummy regression models. I To show how analysis-of-variance models can be handled using dummy variables. c 2014 by John Fox Sociology 740 ° Dummy-Variable Regression and Analysis of Variance 3 3. A Dichotomous Explanatory Variable I The simplest case: one dichotomous and one quantitative explanatory variable. I Assumptions: Relationships are additive — the partial effect of each explanatory • variable is the same regardless of the specific value at which the other explanatory variable is held constant. The other assumptions of the regression model hold. • I The motivation for including a qualitative explanatory variable is the same as for including an additional quantitative explanatory variable: to account more fully for the response variable, by making the errors • smaller; and to avoid a biased assessment of the impact of an explanatory variable, • as a consequence of omitting another explanatory variables that is relatedtoit. -

Analysis of Variance and Analysis of Variance and Design of Experiments of Experiments-I
Analysis of Variance and Design of Experimentseriments--II MODULE ––IVIV LECTURE - 19 EXPERIMENTAL DESIGNS AND THEIR ANALYSIS Dr. Shalabh Department of Mathematics and Statistics Indian Institute of Technology Kanpur 2 Design of experiment means how to design an experiment in the sense that how the observations or measurements should be obtained to answer a qqyuery inavalid, efficient and economical way. The desigggning of experiment and the analysis of obtained data are inseparable. If the experiment is designed properly keeping in mind the question, then the data generated is valid and proper analysis of data provides the valid statistical inferences. If the experiment is not well designed, the validity of the statistical inferences is questionable and may be invalid. It is important to understand first the basic terminologies used in the experimental design. Experimental unit For conducting an experiment, the experimental material is divided into smaller parts and each part is referred to as experimental unit. The experimental unit is randomly assigned to a treatment. The phrase “randomly assigned” is very important in this definition. Experiment A way of getting an answer to a question which the experimenter wants to know. Treatment Different objects or procedures which are to be compared in an experiment are called treatments. Sampling unit The object that is measured in an experiment is called the sampling unit. This may be different from the experimental unit. 3 Factor A factor is a variable defining a categorization. A factor can be fixed or random in nature. • A factor is termed as fixed factor if all the levels of interest are included in the experiment. -

Heteroscedastic Errors
Heteroscedastic Errors ◮ Sometimes plots and/or tests show that the error variances 2 σi = Var(ǫi ) depend on i ◮ Several standard approaches to fixing the problem, depending on the nature of the dependence. ◮ Weighted Least Squares. ◮ Transformation of the response. ◮ Generalized Linear Models. Richard Lockhart STAT 350: Heteroscedastic Errors and GLIM Weighted Least Squares ◮ Suppose variances are known except for a constant factor. 2 2 ◮ That is, σi = σ /wi . ◮ Use weighted least squares. (See Chapter 10 in the text.) ◮ This usually arises realistically in the following situations: ◮ Yi is an average of ni measurements where you know ni . Then wi = ni . 2 ◮ Plots suggest that σi might be proportional to some power of 2 γ γ some covariate: σi = kxi . Then wi = xi− . Richard Lockhart STAT 350: Heteroscedastic Errors and GLIM Variances depending on (mean of) Y ◮ Two standard approaches are available: ◮ Older approach is transformation. ◮ Newer approach is use of generalized linear model; see STAT 402. Richard Lockhart STAT 350: Heteroscedastic Errors and GLIM Transformation ◮ Compute Yi∗ = g(Yi ) for some function g like logarithm or square root. ◮ Then regress Yi∗ on the covariates. ◮ This approach sometimes works for skewed response variables like income; ◮ after transformation we occasionally find the errors are more nearly normal, more homoscedastic and that the model is simpler. ◮ See page 130ff and check under transformations and Box-Cox in the index. Richard Lockhart STAT 350: Heteroscedastic Errors and GLIM Generalized Linear Models ◮ Transformation uses the model T E(g(Yi )) = xi β while generalized linear models use T g(E(Yi )) = xi β ◮ Generally latter approach offers more flexibility. -

Generalized Linear Models
CHAPTER 6 Generalized linear models 6.1 Introduction Generalized linear modeling is a framework for statistical analysis that includes linear and logistic regression as special cases. Linear regression directly predicts continuous data y from a linear predictor Xβ = β0 + X1β1 + + Xkβk.Logistic regression predicts Pr(y =1)forbinarydatafromalinearpredictorwithaninverse-··· logit transformation. A generalized linear model involves: 1. A data vector y =(y1,...,yn) 2. Predictors X and coefficients β,formingalinearpredictorXβ 1 3. A link function g,yieldingavectoroftransformeddataˆy = g− (Xβ)thatare used to model the data 4. A data distribution, p(y yˆ) | 5. Possibly other parameters, such as variances, overdispersions, and cutpoints, involved in the predictors, link function, and data distribution. The options in a generalized linear model are the transformation g and the data distribution p. In linear regression,thetransformationistheidentity(thatis,g(u) u)and • the data distribution is normal, with standard deviation σ estimated from≡ data. 1 1 In logistic regression,thetransformationistheinverse-logit,g− (u)=logit− (u) • (see Figure 5.2a on page 80) and the data distribution is defined by the proba- bility for binary data: Pr(y =1)=y ˆ. This chapter discusses several other classes of generalized linear model, which we list here for convenience: The Poisson model (Section 6.2) is used for count data; that is, where each • data point yi can equal 0, 1, 2, ....Theusualtransformationg used here is the logarithmic, so that g(u)=exp(u)transformsacontinuouslinearpredictorXiβ to a positivey ˆi.ThedatadistributionisPoisson. It is usually a good idea to add a parameter to this model to capture overdis- persion,thatis,variationinthedatabeyondwhatwouldbepredictedfromthe Poisson distribution alone.