Spatial Considerations for Captive Snakes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Traversing Tight Tunnels—Implementing an Adaptive Concertina Gait in a Biomimetic Snake Robot Henry C
Earth and Space 2018 158 Traversing Tight Tunnels—Implementing an Adaptive Concertina Gait in a Biomimetic Snake Robot Henry C. Astley1 1Biomimicry Research and Innovation Center, Dept. of Biology and Polymer Science, Univ. of Akron, 235 Carroll St., Akron, OH 44325-3908. E-mail: [email protected] ABSTRACT Snakes move through cluttered habitats and tight spaces with extraordinary ease, and consequently snake robots are a popular design for such situations. The remarkable locomotor performance of snakes is due in part to their diversity of locomotor modes for addressing a range of environmental challenges. Concertina locomotion consists of alternating periods of static anchoring and movement along the snake’s body, and is used to negotiate narrow spaces such as tunnels or bare branches. However, concertina locomotion has rarely been implemented in snake robots. In this paper, the anchor formation process in live snakes during concertina locomotion is quantified and used to implement concertina locomotion in a snake robot, with automatic detection of the width of the tunnel walls to modulate the waveform. This allows effective concertina locomotion in tunnels of unknown width, without prior knowledge of tunnel geometry, expanding the range of gaits in snake robots. INTRODUCTION The elongate, limbless body plan is extremely common throughout nature, in both vertebrate and invertebrates alike (Pechenik, J. A. 2005; Pough, F. H. et al. 2002), and has evolved independently numerous times (Gans, C 1975). Indeed, within lizards (Order Squamata) alone there are over two dozen independent evolutions of limblessness or functional limblessness (Wiens, J. J. et al. 2006). Snakes are by far the most successful limbless taxon, with a near-global distribution, nearly 3000 species, and a wide range of ecological niches and habitats (Pough, F. -

Slithering Locomotion
SLITHERING LOCOMOTION DAVID L. HU() AND MICHAEL SHELLEY∗∗ Abstract. Limbless terrestrial animals propel themselves by sliding their bellies along the ground. Although the study of dry solid-solid friction is a classical subject, the mechanisms underlying friction-based limbless propulsion have received little attention. We review and expand upon our previous work on the locomotion of snakes, who are expert sliders. We show that snakes use two principal mechanisms to slither on flat surfaces. First, their bellies are covered with scales that catch upon ground asperities, providing frictional anisotropy. Second, they are able to lift parts of their body slightly off the ground when moving. This reduces undesired frictional drag and applies greater pressure to the parts of the belly that are pushing the snake forwards. We review a theoretical framework that may be adapted by future investigators to understand other kinds of limbless locomotion. Key words. Snakes, friction, locomotion AMS(MOS) subject classifications. Primary 76Zxx 1. Introduction. Animal locomotion is as diverse as animal form. Swimming, flying and walking have received much attention [1, 9]withthe latter being the most commonly studied means for moving on land (Fig. 1). Comparatively little attention has been paid to limbless locomotion on land, which necessarily relies upon sliding. Sliding is physically distinct from pushing against a fluid and understanding it as a form of locomotion presents new challenges, as we present in this review. Terrestrial limbless animals are rare. Those that are multicellular include worms, snails and snakes, and account for less than 2% of the 1.8 million named species (Fig. -

Quick Reference Guide: Introduced Constrictors in Florida1 Steve A
WEC302 Quick Reference Guide: Introduced Constrictors in Florida1 Steve A. Johnson and Monica E. McGarrity2 Three non-native species of large constrictor snakes are that these were escaped or released pets. View maps of loca- now breeding in Florida, and several others have been tions where each species has been encountered in Florida encountered but have not yet established wild populations. by visiting the EDDMapS Florida invasive species reporting This fact sheet, best viewed as a pdf (http://edis.ifas.ufl.edu/ portal online at http://www.IveGot1.org. Learn more about pdffiles/UW/UW34700.pdf), is a quick reference guide how to scan for, recognize, and report introduced constric- to identification of the constrictors you are most likely to tors by completing the Introduced Reptile Early Detection encounter in Florida. Although many of these snakes are and Documentation training course. Visit http://ufwildlife. not established in the wild, they are common in the pet ifas.ufl.edu/reddy.shtml to learn more and get REDDy! trade, and each has been spotted in the wild—it is likely Pythons Burmese Python (Python bivittatus) Status: established, breeding populations; range expanding Head: dark arrowhead, light center line, dark and light in Florida wedges under eyes Size: up to 12 feet or longer Body: Giraffe-like spots, dark blotches not connected Figure 1. Burmese python. Credits: Head illustration by USGS; body illustration by Monica E. McGarrity, UF 1. This document is WEC302, one of a series of the Department of Wildlife Ecology and Conservation, UF/IFAS Extension. Original publication date November 2010. Revised February 2014 and June 2017. -

A New Species of Hepatozoon (Apicomplexa: Adeleorina) from Python Regius (Serpentes: Pythonidae) and Its Experimental Transmission by a Mosquito Vector
J. Parasitol., 93(?), 2007, pp. 1189–1198 ᭧ American Society of Parasitologists 2007 A NEW SPECIES OF HEPATOZOON (APICOMPLEXA: ADELEORINA) FROM PYTHON REGIUS (SERPENTES: PYTHONIDAE) AND ITS EXPERIMENTAL TRANSMISSION BY A MOSQUITO VECTOR Michal Sloboda, Martin Kamler, Jana Bulantova´*, Jan Voty´pka*†, and David Modry´† Department of Parasitology, University of Veterinary and Pharmaceutical Sciences, Palacke´ho 1-3, 612 42 Brno, Czech Republic. e-mail: [email protected] ABSTRACT: Hepatozoon ayorgbor n. sp. is described from specimens of Python regius imported from Ghana. Gametocytes were found in the peripheral blood of 43 of 55 snakes examined. Localization of gametocytes was mainly inside the erythrocytes; free gametocytes were found in 15 (34.9%) positive specimens. Infections of laboratory-reared Culex quinquefasciatus feeding on infected snakes, as well as experimental infection of juvenile Python regius by ingestion of infected mosquitoes, were performed to complete the life cycle. Similarly, transmission to different snake species (Boa constrictor and Lamprophis fuliginosus) and lizards (Lepidodactylus lugubris) was performed to assess the host specificity. Isolates were compared with Hepatozoon species from sub-Saharan reptiles and described as a new species based on the morphology, phylogenetic analysis, and a complete life cycle. Hemogregarines are the most common intracellular hemo- 3 genera (Telford et al., 2004). Low host specificity of Hepa- parasites found in reptiles. The Hemogregarinidae, Karyolysi- tozoon spp. is supported by experimental transmissions between dae, and Hepatozoidae are distinguished based on the different snakes from different families. Ball (1967) observed experi- developmental patterns in definitive (invertebrate) hosts oper- mental parasitemia with Hepatozoon rarefaciens in the Boa ating as vectors; all 3 families have heteroxenous life cycles constrictor (Boidae); the vector was Culex tarsalis, which had (Telford, 1984). -

Dispelling Python Regius Myths "Gorzula, Stefan, William Owusu Nsiah, and William Oduro
Dispelling Python regius Myths "Gorzula, Stefan, William Owusu Nsiah, and William Oduro. Survey of the status and By Francis Cosquieri management of the Royal Python (Python regius) in Ghana. Secrétariat CITES, 1997." This paper lists several pythons being found in Before we get deep into the “nitty gritty” of the trees, although points out that the species is subject I want to take a moment to say that the very adaptable to the point of being AHH Royal Python sub-group "Not Just a Pet semi-invasive and responds well to Rock" is the best place to start for getting rid of anthropogenic disturbance. the preconceptions surrounding the poor old Royal Python. Zack Tippie and the other admins have done an incredible job of "Luiselli, Luca, and Francesco Maria Angelici. dispelling the myths surrounding Royal Python "Sexual size dimorphism and natural history behaviour and husbandry, and Zack’s care traits are correlated with intersexual dietary guide for this oft-maligned snake (to be found divergence in royal pythons (Python regius) in the third Advancing Herpetological from the rainforests of southeastern Nigeria." Husbandry Quarterly Newsletter) is one of the Italian Journal of Zoology 65.2 (1998): best I have read. 183-185." - In the meantime, here is a post I made some Half of the male pythons encountered over a time ago on AHH itself bringing together some two year period were found on trees, there is a information on the wild habits of this python. large discrepancy between habitat use by All the papers mentioned here can be found in male and female pythons, and by larger and the AHH Files section. -

Investigations Into the Presence of Nidoviruses in Pythons Silvia Blahak1, Maria Jenckel2,3, Dirk Höper2, Martin Beer2, Bernd Hoffmann2 and Kore Schlottau2*
Blahak et al. Virology Journal (2020) 17:6 https://doi.org/10.1186/s12985-020-1279-5 RESEARCH Open Access Investigations into the presence of nidoviruses in pythons Silvia Blahak1, Maria Jenckel2,3, Dirk Höper2, Martin Beer2, Bernd Hoffmann2 and Kore Schlottau2* Abstract Background: Pneumonia and stomatitis represent severe and often fatal diseases in different captive snakes. Apart from bacterial infections, paramyxo-, adeno-, reo- and arenaviruses cause these diseases. In 2014, new viruses emerged as the cause of pneumonia in pythons. In a few publications, nidoviruses have been reported in association with pneumonia in ball pythons and a tiger python. The viruses were found using new sequencing methods from the organ tissue of dead animals. Methods: Severe pneumonia and stomatitis resulted in a high mortality rate in a captive breeding collection of green tree pythons. Unbiased deep sequencing lead to the detection of nidoviral sequences. A developed RT-qPCR was used to confirm the metagenome results and to determine the importance of this virus. A total of 1554 different boid snakes, including animals suffering from respiratory diseases as well as healthy controls, were screened for nidoviruses. Furthermore, in addition to two full-length sequences, partial sequences were generated from different snake species. Results: The assembled full-length snake nidovirus genomes share only an overall genome sequence identity of less than 66.9% to other published snake nidoviruses and new partial sequences vary between 99.89 and 79.4%. Highest viral loads were detected in lung samples. The snake nidovirus was not only present in diseased animals, but also in snakes showing no typical clinical signs. -

CNN-Based Genetic Algorithm
International Journal of Computational Intelligence Systems, Vol.2, No. 2 (June, 2009), 124-131 Cellular Neural Networks-Based Genetic Algorithm for Optimizing the Behavior of an Unstructured Robot Alireza Fasih Transportation Informatics Group, Institute of Smart Systems Technologies, University of Klagenfurt Klagenfurt, Austria E-mail: [email protected] Jean Chamberlain Chedjou Transportation Informatics Group, Institute of Smart Systems Technologies, University of Klagenfurt E-mail: [email protected] Kyandoghere Kyamakya Transportation Informatics Group, Institute of Smart Systems Technologies, University of Klagenfurt E-mail: [email protected] Abstract A new learning algorithm for advanced robot locomotion is presented in this paper. This method involves both Cellular Neural Networks (CNN) technology and an evolutionary process based on genetic algorithm (GA) for a learning process. Learning is formulated as an optimization problem. CNN Templates are derived by GA after an optimization process. Through these templates the CNN computation platform generates a specific wave leading to the best motion of a walker robot. It is demonstrated that due to the new method presented in this paper an irregular and even a disjointed walker robot can successfully move with the highest performance. Keywords: Cellular Neural Networks, Robot locomotion, Simulation, Genetic Algorithms. 1. Introduction of animal walking motion with the aim of optimizing the energy consumption [2-4]. It is well-known that the Nowadays, some of the main goals of robotics science, walking motion of animals is of a stereotype. In a large mechatronics and artificial intelligence lie in designing variety of animals a central neural controller does mechanisms close to or mimicking as good as possible organize/coordinate the motion. -

Ball Python Care Sheet
Ball Python Care Sheet NATURAL HISTORY: The Ball Python (also called the Royal Python) is by far the most common pet python in this part of the world. Ball pythons are native to sub-Saharan West Africa and thrive in extreme heat and humidity. Ball pythons are generally hardy and tend to do well in captivity if all of their basic husbandry needs are met. They are friendly, easy to handle, and a bit shy — making them a favorite amongst many reptile owners. Males range in size from 2-3 feet and females 3-5 feet. A ball python’s lifespan is variable, however, they can live for up to 25-35 years in captivity. DIET: Ball pythons consume rodents exclusively. Ball pythons can be fed mice or rats depending on the age and size of the snake. As a rule of thumb, the size (largest diameter) of the prey item should be approximately the same diameter as the snake’s body. Feeder rodents can be purchased alive or frozen. I recommend feeding frozen-thawed or pre-killed rodents to all pet snakes because live prey items are able to fight back. Keep in mind that rodents have very strong jaws, with sharp teeth, and are capable of inflicting serious wounds on your pet. Therefore, a live rodent should never be left in a snake’s enclosure without direct supervision. Some people prefer to feed snakes in a separate bin or tank (outside of their primary enclosure) which may help prevent accidental bites while reaching into their primary enclosure. Juvenile ball pythons should be fed weekly and adults may be fed bi-weekly or monthly depending on the size of the prey item. -
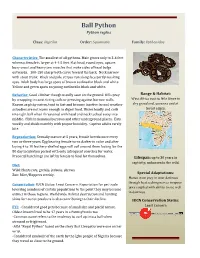
Ball Python Python Regius
Ball Python Python regius Class: Reptilia Order: Squamata Family: Pythonidae Characteristics: The smallest of all pythons. Male grows only to 3-4 feet whereas female is larger at 4-4.5 feet. Flat head, round eyes, square, boxy snout and heavy jaw muscles that make sides of head bulge outwards. 100-150 sharp teeth curve toward the back. Neck narrow with stout trunk. Black and pale stripes run along face partly masking eyes. Adult body has large spots of brown outlined in black and white. Yellow and green spots on young outlined in black and white. Behavior: Good climber though usually seen on the ground. Kills prey Range & Habitat: by wrapping in constricting coils or pressing against burrow walls. West Africa east to Nile River in Known as picky eaters, tend to fast and become inactive in cool weather dry grassland, savanna and at as bodies are not warm enough to digest food. Hisses loudly and coils forest edges. into tight ball when threatened with head and neck tucked away into middle. Hide in mammal burrows and other underground places. Eats weekly and sheds monthly with proper humidity. Captive adults rarely bite. Reproduction: Sexually mature at 5 years, female breeds once every two or three years. Egg bearing female turns darker in color and after laying 4 to 10 leathery-shelled eggs will coil around them fasting for the 80 day incubation period with only infrequent searches for water. Precocial hatchlings are left by female to fend for themselves. Lifespan: up to 30 years in captivity, unknown in the wild. -

THE HUSBANDRY and CAPTIVE PROPAGATION of the SOUTHERN ROCK PYTHON, PYTHON SEBAE NATALENSIS GERALD V HAAGNER Port Elizabeth Snake Park, P.O
BIMCI) I lerpciolopt.)1 Sotici.1 Both:NIL No. 42. 1992/93. THE HUSBANDRY AND CAPTIVE PROPAGATION OF THE SOUTHERN ROCK PYTHON, PYTHON SEBAE NATALENSIS GERALD V HAAGNER Port Elizabeth Snake Park, P.O. Box 13147 Hum wood, 6013. South Africa INTRODUCTION The African Rock Python, Python sebae (Fig. I) is distributed throughout much of sub-Saharan Africa, extending along the Nile valley into Egypt. In southern Africa it is restricted to northern Namibia and Botswana, extending through the northern and eastern Tansvaal, to Natal and the Transkei (Map 1). Pythons were previously found in the eastern Cape Province as far south as Bathurst where it became extinct in 1927 (Broadley, 1983). Branch (1988a) reported on an apparent successful reintroduction of the species into the eastern Cape. Broadley (1984) recently recognised the southern race (Python sebac natalensis) of the African Rock Python as distinct from the typical race from west and central Africa. The recently described Lesser Rock Python (P. saxuloides) from Kenya (Miller & Smith 1979), is treated as a junior synonym of the southern race (Broadley 1983). The African Rock Python is a thick-bodied snake with distinctive markings and size. As with most "giant" snakes. the size is easily over-exaggerated and most recorded lengths have been of the nominate race. Python sebae sebae has been reported to attain a length of 9.8m in the Ivory Coast (Doucet 1963), but full details for this specimen are lacking and cannot now be verified. Arthur Loveridge, whilst collecting in East Africa in 1927, measured a fresh skin of 9.1 metres and even allowing for extensive stretching, it must have been over seven metres long. -

Muscular Mechanisms and Kinematics of Rectilinear Locomotion in Boa Constrictors Steven J
© 2018. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2018) 221, jeb166199. doi:10.1242/jeb.166199 RESEARCH ARTICLE Crawling without wiggling: muscular mechanisms and kinematics of rectilinear locomotion in boa constrictors Steven J. Newman and Bruce C. Jayne* ABSTRACT how such animals coordinate the muscle activity and movement A central issue for understanding locomotion of vertebrates is how among all of these serially homologous structures. Within muscle activity and movements of their segmented axial structures are vertebrates, both lampreys and snakes have body segments with coordinated, and snakes have a longitudinal uniformity of body size and shape that are relatively uniform along most of their length, segments and diverse locomotor behaviors that are well suited for and the absence of appendages provides a somewhat simplified studying the neural control of rhythmic axial movements. Unlike all body plan that makes both of these groups well suited for studying other major modes of snake locomotion, rectilinear locomotion does the neural control of rhythmic axial movements (Cohen, 1988). not involve axial bending, and the mechanisms of propulsion and Furthermore, snakes use different modes of locomotion in a variety modulating speed are not well understood. We integrated of environments, which facilitates investigating the diversity and electromyograms and kinematics of boa constrictors to test plasticity of axial motor patterns. Lissmann’s decades-old hypotheses of activity of the Most previous studies recognize at least four major modes of costocutaneous superior (CCS) and inferior (CCI) muscles and the terrestrial snake locomotion (Mosauer, 1932; Gray, 1968; Gans, intrinsic cutaneous interscutalis (IS) muscle during rectilinear 1974; Jayne, 1986; Cundall, 1987), but rectilinear is the only one of locomotion. -

Alexander 2013 Principles-Of-Animal-Locomotion.Pdf
.................................................... Principles of Animal Locomotion Principles of Animal Locomotion ..................................................... R. McNeill Alexander PRINCETON UNIVERSITY PRESS PRINCETON AND OXFORD Copyright © 2003 by Princeton University Press Published by Princeton University Press, 41 William Street, Princeton, New Jersey 08540 In the United Kingdom: Princeton University Press, 3 Market Place, Woodstock, Oxfordshire OX20 1SY All Rights Reserved Second printing, and first paperback printing, 2006 Paperback ISBN-13: 978-0-691-12634-0 Paperback ISBN-10: 0-691-12634-8 The Library of Congress has cataloged the cloth edition of this book as follows Alexander, R. McNeill. Principles of animal locomotion / R. McNeill Alexander. p. cm. Includes bibliographical references (p. ). ISBN 0-691-08678-8 (alk. paper) 1. Animal locomotion. I. Title. QP301.A2963 2002 591.47′9—dc21 2002016904 British Library Cataloging-in-Publication Data is available This book has been composed in Galliard and Bulmer Printed on acid-free paper. ∞ pup.princeton.edu Printed in the United States of America 1098765432 Contents ............................................................... PREFACE ix Chapter 1. The Best Way to Travel 1 1.1. Fitness 1 1.2. Speed 2 1.3. Acceleration and Maneuverability 2 1.4. Endurance 4 1.5. Economy of Energy 7 1.6. Stability 8 1.7. Compromises 9 1.8. Constraints 9 1.9. Optimization Theory 10 1.10. Gaits 12 Chapter 2. Muscle, the Motor 15 2.1. How Muscles Exert Force 15 2.2. Shortening and Lengthening Muscle 22 2.3. Power Output of Muscles 26 2.4. Pennation Patterns and Moment Arms 28 2.5. Power Consumption 31 2.6. Some Other Types of Muscle 34 Chapter 3.