Review Article Current View on Phytoplasma Genomes and Encoded Metabolism
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Progressive Increase in Mtdna 3243A>G Heteroplasmy Causes Abrupt
Progressive increase in mtDNA 3243A>G PNAS PLUS heteroplasmy causes abrupt transcriptional reprogramming Martin Picarda, Jiangwen Zhangb, Saege Hancockc, Olga Derbenevaa, Ryan Golhard, Pawel Golike, Sean O’Hearnf, Shawn Levyg, Prasanth Potluria, Maria Lvovaa, Antonio Davilaa, Chun Shi Lina, Juan Carlos Perinh, Eric F. Rappaporth, Hakon Hakonarsonc, Ian A. Trouncei, Vincent Procaccioj, and Douglas C. Wallacea,1 aCenter for Mitochondrial and Epigenomic Medicine, Children’s Hospital of Philadelphia and the Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA 19104; bSchool of Biological Sciences, The University of Hong Kong, Hong Kong, People’s Republic of China; cTrovagene, San Diego, CA 92130; dCenter for Applied Genomics, Division of Genetics, Department of Pediatrics, and hNucleic Acid/Protein Research Core Facility, Children’s Hospital of Philadelphia, Philadelphia, PA 19104; eInstitute of Genetics and Biotechnology, Warsaw University, 00-927, Warsaw, Poland; fMorton Mower Central Research Laboratory, Sinai Hospital of Baltimore, Baltimore, MD 21215; gGenomics Sevices Laboratory, HudsonAlpha Institute for Biotechnology, Huntsville, AL 35806; iCentre for Eye Research Australia, Royal Victorian Eye and Ear Hospital, East Melbourne, VIC 3002, Australia; and jDepartment of Biochemistry and Genetics, National Center for Neurodegenerative and Mitochondrial Diseases, Centre Hospitalier Universitaire d’Angers, 49933 Angers, France Contributed by Douglas C. Wallace, August 1, 2014 (sent for review May -

Bacterial Communities of the Upper Respiratory Tract of Turkeys
www.nature.com/scientificreports OPEN Bacterial communities of the upper respiratory tract of turkeys Olimpia Kursa1*, Grzegorz Tomczyk1, Anna Sawicka‑Durkalec1, Aleksandra Giza2 & Magdalena Słomiany‑Szwarc2 The respiratory tracts of turkeys play important roles in the overall health and performance of the birds. Understanding the bacterial communities present in the respiratory tracts of turkeys can be helpful to better understand the interactions between commensal or symbiotic microorganisms and other pathogenic bacteria or viral infections. The aim of this study was the characterization of the bacterial communities of upper respiratory tracks in commercial turkeys using NGS sequencing by the amplifcation of 16S rRNA gene with primers designed for hypervariable regions V3 and V4 (MiSeq, Illumina). From 10 phyla identifed in upper respiratory tract in turkeys, the most dominated phyla were Firmicutes and Proteobacteria. Diferences in composition of bacterial diversity were found at the family and genus level. At the genus level, the turkey sequences present in respiratory tract represent 144 established bacteria. Several respiratory pathogens that contribute to the development of infections in the respiratory system of birds were identifed, including the presence of Ornithobacterium and Mycoplasma OTUs. These results obtained in this study supply information about bacterial composition and diversity of the turkey upper respiratory tract. Knowledge about bacteria present in the respiratory tract and the roles they can play in infections can be useful in controlling, diagnosing and treating commercial turkey focks. Next-generation sequencing has resulted in a marked increase in culture-independent studies characterizing the microbiome of humans and animals1–6. Much of these works have been focused on the gut microbiome of humans and other production animals 7–11. -

Global Metagenomic Survey Reveals a New Bacterial Candidate Phylum in Geothermal Springs
ARTICLE Received 13 Aug 2015 | Accepted 7 Dec 2015 | Published 27 Jan 2016 DOI: 10.1038/ncomms10476 OPEN Global metagenomic survey reveals a new bacterial candidate phylum in geothermal springs Emiley A. Eloe-Fadrosh1, David Paez-Espino1, Jessica Jarett1, Peter F. Dunfield2, Brian P. Hedlund3, Anne E. Dekas4, Stephen E. Grasby5, Allyson L. Brady6, Hailiang Dong7, Brandon R. Briggs8, Wen-Jun Li9, Danielle Goudeau1, Rex Malmstrom1, Amrita Pati1, Jennifer Pett-Ridge4, Edward M. Rubin1,10, Tanja Woyke1, Nikos C. Kyrpides1 & Natalia N. Ivanova1 Analysis of the increasing wealth of metagenomic data collected from diverse environments can lead to the discovery of novel branches on the tree of life. Here we analyse 5.2 Tb of metagenomic data collected globally to discover a novel bacterial phylum (‘Candidatus Kryptonia’) found exclusively in high-temperature pH-neutral geothermal springs. This lineage had remained hidden as a taxonomic ‘blind spot’ because of mismatches in the primers commonly used for ribosomal gene surveys. Genome reconstruction from metagenomic data combined with single-cell genomics results in several high-quality genomes representing four genera from the new phylum. Metabolic reconstruction indicates a heterotrophic lifestyle with conspicuous nutritional deficiencies, suggesting the need for metabolic complementarity with other microbes. Co-occurrence patterns identifies a number of putative partners, including an uncultured Armatimonadetes lineage. The discovery of Kryptonia within previously studied geothermal springs underscores the importance of globally sampled metagenomic data in detection of microbial novelty, and highlights the extraordinary diversity of microbial life still awaiting discovery. 1 Department of Energy Joint Genome Institute, Walnut Creek, California 94598, USA. 2 Department of Biological Sciences, University of Calgary, Calgary, Alberta T2N 1N4, Canada. -

MIB–MIP Is a Mycoplasma System That Captures and Cleaves Immunoglobulin G
MIB–MIP is a mycoplasma system that captures and cleaves immunoglobulin G Yonathan Arfia,b,1, Laetitia Minderc,d, Carmelo Di Primoe,f,g, Aline Le Royh,i,j, Christine Ebelh,i,j, Laurent Coquetk, Stephane Claveroll, Sanjay Vasheem, Joerg Joresn,o, Alain Blancharda,b, and Pascal Sirand-Pugneta,b aINRA (Institut National de la Recherche Agronomique), UMR 1332 Biologie du Fruit et Pathologie, F-33882 Villenave d’Ornon, France; bUniversity of Bordeaux, UMR 1332 Biologie du Fruit et Pathologie, F-33882 Villenave d’Ornon, France; cInstitut Européen de Chimie et Biologie, UMS 3033, University of Bordeaux, 33607 Pessac, France; dInstitut Bergonié, SIRIC BRIO, 33076 Bordeaux, France; eINSERM U1212, ARN Regulation Naturelle et Artificielle, 33607 Pessac, France; fCNRS UMR 5320, ARN Regulation Naturelle et Artificielle, 33607 Pessac, France; gInstitut Européen de Chimie et Biologie, University of Bordeaux, 33607 Pessac, France; hInstitut de Biologie Structurale, University of Grenoble Alpes, F-38044 Grenoble, France; iCNRS, Institut de Biologie Structurale, F-38044 Grenoble, France; jCEA, Institut de Biologie Structurale, F-38044 Grenoble, France; kCNRS UMR 6270, Plateforme PISSARO, Institute for Research and Innovation in Biomedicine - Normandie Rouen, Normandie Université, F-76821 Mont-Saint-Aignan, France; lProteome Platform, Functional Genomic Center of Bordeaux, University of Bordeaux, F-33076 Bordeaux Cedex, France; mJ. Craig Venter Institute, Rockville, MD 20850; nInternational Livestock Research Institute, 00100 Nairobi, Kenya; and oInstitute of Veterinary Bacteriology, University of Bern, CH-3001 Bern, Switzerland Edited by Roy Curtiss III, University of Florida, Gainesville, FL, and approved March 30, 2016 (received for review January 12, 2016) Mycoplasmas are “minimal” bacteria able to infect humans, wildlife, introduced into naive herds (8). -

Supplementary Information for Microbial Electrochemical Systems Outperform Fixed-Bed Biofilters for Cleaning-Up Urban Wastewater
Electronic Supplementary Material (ESI) for Environmental Science: Water Research & Technology. This journal is © The Royal Society of Chemistry 2016 Supplementary information for Microbial Electrochemical Systems outperform fixed-bed biofilters for cleaning-up urban wastewater AUTHORS: Arantxa Aguirre-Sierraa, Tristano Bacchetti De Gregorisb, Antonio Berná, Juan José Salasc, Carlos Aragónc, Abraham Esteve-Núñezab* Fig.1S Total nitrogen (A), ammonia (B) and nitrate (C) influent and effluent average values of the coke and the gravel biofilters. Error bars represent 95% confidence interval. Fig. 2S Influent and effluent COD (A) and BOD5 (B) average values of the hybrid biofilter and the hybrid polarized biofilter. Error bars represent 95% confidence interval. Fig. 3S Redox potential measured in the coke and the gravel biofilters Fig. 4S Rarefaction curves calculated for each sample based on the OTU computations. Fig. 5S Correspondence analysis biplot of classes’ distribution from pyrosequencing analysis. Fig. 6S. Relative abundance of classes of the category ‘other’ at class level. Table 1S Influent pre-treated wastewater and effluents characteristics. Averages ± SD HRT (d) 4.0 3.4 1.7 0.8 0.5 Influent COD (mg L-1) 246 ± 114 330 ± 107 457 ± 92 318 ± 143 393 ± 101 -1 BOD5 (mg L ) 136 ± 86 235 ± 36 268 ± 81 176 ± 127 213 ± 112 TN (mg L-1) 45.0 ± 17.4 60.6 ± 7.5 57.7 ± 3.9 43.7 ± 16.5 54.8 ± 10.1 -1 NH4-N (mg L ) 32.7 ± 18.7 51.6 ± 6.5 49.0 ± 2.3 36.6 ± 15.9 47.0 ± 8.8 -1 NO3-N (mg L ) 2.3 ± 3.6 1.0 ± 1.6 0.8 ± 0.6 1.5 ± 2.0 0.9 ± 0.6 TP (mg -

Branched Chain A-Ketoacid Dehydrogenase to Thiamine and Thiamine Pyrophosphate
Pediat. Res. 12: 235-238 (1978) Branched chain amino acids thiamine maple syrup urine disease vitamin responsiveness mitochondrial membranes In Vivo and in Vitro Response of Human Branched Chain a-Ketoacid Dehydrogenase to Thiamine and Thiamine Pyrophosphate DEAN J. DANNER,'27' FRANCES B. WHEELER, SANDRA K. LEMMON, AND LOUIS J. ELSAS I1 Department of Pediatrics, Division of Medical Genetics, Emory University School of Medicine, Atlanta, Georgia, USA Summary lability at 37"; and failure to respond to added NAD+, CoASH, and MgZ+. In a homozygous affected patient with maple syrup urine We propose that "excess" thiamine led to increased available disease, pharmacologic doses of thiamine lowered urinary excre- thiamine pyrophosphate which stabilized the branched chain a- tion of branched chain a-ketoacids and stimulated branched ketoacid dehydrogenase, decreased biologic turnover, increased chain a-ketoacid dehydrogenase (BCKAD) in his peripheral enzyme specific activity and produced in vivo tolerance to blood leukocvtes. Suvvlementation of his branched chain ami- branched chain aminoacids in these patients with maple syrup noacid restricted diei hth 100 mglday of thiamine eliminated urine disease. recurrent episodes of ketoacidosis.~heseclinical responses were Speculation studied in vitro using mitochondria1 inner membranes prepared from his cultured skin fibroblasts and those from another By studying the partially purified normal and mutant branched thiamine-responsive patient from Canada. BCKAD in both chain a-ketoacid dehydrogenases from cultured human fibro- mutant cell lines had similarities to normal enzyme including: blasts, direct in vitro effects of thiamine pyrophosphate can be identical apparent K,,, value for thiamine pyrophosphate; similar measured and related to in vivo clinical responses. -

Supplementary Information
Supplementary information (a) (b) Figure S1. Resistant (a) and sensitive (b) gene scores plotted against subsystems involved in cell regulation. The small circles represent the individual hits and the large circles represent the mean of each subsystem. Each individual score signifies the mean of 12 trials – three biological and four technical. The p-value was calculated as a two-tailed t-test and significance was determined using the Benjamini-Hochberg procedure; false discovery rate was selected to be 0.1. Plots constructed using Pathway Tools, Omics Dashboard. Figure S2. Connectivity map displaying the predicted functional associations between the silver-resistant gene hits; disconnected gene hits not shown. The thicknesses of the lines indicate the degree of confidence prediction for the given interaction, based on fusion, co-occurrence, experimental and co-expression data. Figure produced using STRING (version 10.5) and a medium confidence score (approximate probability) of 0.4. Figure S3. Connectivity map displaying the predicted functional associations between the silver-sensitive gene hits; disconnected gene hits not shown. The thicknesses of the lines indicate the degree of confidence prediction for the given interaction, based on fusion, co-occurrence, experimental and co-expression data. Figure produced using STRING (version 10.5) and a medium confidence score (approximate probability) of 0.4. Figure S4. Metabolic overview of the pathways in Escherichia coli. The pathways involved in silver-resistance are coloured according to respective normalized score. Each individual score represents the mean of 12 trials – three biological and four technical. Amino acid – upward pointing triangle, carbohydrate – square, proteins – diamond, purines – vertical ellipse, cofactor – downward pointing triangle, tRNA – tee, and other – circle. -

Data Sheet on Spiroplasma Citri
Prepared by CABI and EPPO for the EU under Contract 90/399003 Data Sheets on Quarantine Pests Spiroplasma citri The vectors of Spiroplasma citri are individually included in EU Directive 77/93. Since their importance only arises in relation to S. citri, they are covered in this data sheet. IDENTITY • Spiroplasma citri Name: Spiroplasma citri Saglio et al. Taxonomic position: Bacteria: Tenericutes: Mollicutes Common names: Stubborn, little leaf (English) Stubborn (French) Bayer computer code: SPIRCI EU Annex designation: II/A2 • Circulifer tenellus Name: Circulifer tenellus (Baker) Synonyms: Neoaliturus tenellus (Baker) Eutettix tenellus Baker Taxonomic position: Insecta: Hemiptera: Homoptera: Cicadellidae Common names: Beet leafhopper (English) Cicadelle de la betterave (French) Saltahojas de la remolacha (Spanish) Notes on taxonomy and nomenclature: Oman (1970) argues for the retention of Circulifer and Neoaliturus as separate genera; in his concept both tenellus and haematoceps are placed in Circulifer. Nast (1972) treats the two genera as Neoaliturus notwithstanding Oman's (1970) arguments for the retention of the two genera. He includes 17 species in the Palaearctic region of which 14 are recorded from the Mediterranean Basin. The two species, tenellus and haematoceps, are both included in Circulifer by della Giustina (1989), who reports that tenellus is confirmed in Corsica. Bayer computer code: CIRCTE EU Annex designation: II/A2 • Neoaliturus haematoceps Name: Neoaliturus haematoceps (Mulsant & Rey) Synonyms: Circulifer haematoceps (Mulsant & Rey) Jassus haematoceps Mulsant & Rey Taxonomic position: Insecta: Hemiptera: Homoptera: Cicadellidae Bayer computer code: NEOAHA EU Annex designation: II/A2 (under the name Circulifer haematoceps) HOSTS • Spiroplasma citri The principal economic hosts of S. citri are susceptible Citrus spp. -

Downloaded from Genome Website
bioRxiv preprint doi: https://doi.org/10.1101/2020.11.18.388454; this version posted November 19, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Characterization of the first cultured free-living representative of 2 Candidatus Izimaplasma uncovers its unique biology 3 Rikuan Zheng1,2,3,4, Rui Liu1,2,4, Yeqi Shan1,2,3,4, Ruining Cai1,2,3,4, Ge Liu1,2,4, Chaomin Sun1,2,4* 1 4 CAS Key Laboratory of Experimental Marine Biology & Center of Deep Sea 5 Research, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China 2 6 Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory 7 for Marine Science and Technology, Qingdao, China 3 8 College of Earth Science, University of Chinese Academy of Sciences, Beijing, 9 China 10 4Center of Ocean Mega-Science, Chinese Academy of Sciences, Qingdao, China 11 12 * Corresponding author 13 Chaomin Sun Tel.: +86 532 82898857; fax: +86 532 82898857. 14 E-mail address: [email protected] 15 16 17 Key words: Candidatus Izimaplasma, uncultivation, biogeochemical cycling, 18 extracellular DNA, in situ, deep sea 19 Running title: Characterization of the first cultured Izimaplasma 20 21 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.11.18.388454; this version posted November 19, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 22 Abstract 23 Candidatus Izimaplasma, an intermediate in the reductive evolution from Firmicutes 24 to Mollicutes, was proposed to represent a novel class of free-living wall-less bacteria 25 within the phylum Tenericutes found in deep-sea methane seeps. -

Supplementary Informations SI2. Supplementary Table 1
Supplementary Informations SI2. Supplementary Table 1. M9, soil, and rhizosphere media composition. LB in Compound Name Exchange Reaction LB in soil LBin M9 rhizosphere H2O EX_cpd00001_e0 -15 -15 -10 O2 EX_cpd00007_e0 -15 -15 -10 Phosphate EX_cpd00009_e0 -15 -15 -10 CO2 EX_cpd00011_e0 -15 -15 0 Ammonia EX_cpd00013_e0 -7.5 -7.5 -10 L-glutamate EX_cpd00023_e0 0 -0.0283302 0 D-glucose EX_cpd00027_e0 -0.61972444 -0.04098397 0 Mn2 EX_cpd00030_e0 -15 -15 -10 Glycine EX_cpd00033_e0 -0.0068175 -0.00693094 0 Zn2 EX_cpd00034_e0 -15 -15 -10 L-alanine EX_cpd00035_e0 -0.02780553 -0.00823049 0 Succinate EX_cpd00036_e0 -0.0056245 -0.12240603 0 L-lysine EX_cpd00039_e0 0 -10 0 L-aspartate EX_cpd00041_e0 0 -0.03205557 0 Sulfate EX_cpd00048_e0 -15 -15 -10 L-arginine EX_cpd00051_e0 -0.0068175 -0.00948672 0 L-serine EX_cpd00054_e0 0 -0.01004986 0 Cu2+ EX_cpd00058_e0 -15 -15 -10 Ca2+ EX_cpd00063_e0 -15 -100 -10 L-ornithine EX_cpd00064_e0 -0.0068175 -0.00831712 0 H+ EX_cpd00067_e0 -15 -15 -10 L-tyrosine EX_cpd00069_e0 -0.0068175 -0.00233919 0 Sucrose EX_cpd00076_e0 0 -0.02049199 0 L-cysteine EX_cpd00084_e0 -0.0068175 0 0 Cl- EX_cpd00099_e0 -15 -15 -10 Glycerol EX_cpd00100_e0 0 0 -10 Biotin EX_cpd00104_e0 -15 -15 0 D-ribose EX_cpd00105_e0 -0.01862144 0 0 L-leucine EX_cpd00107_e0 -0.03596182 -0.00303228 0 D-galactose EX_cpd00108_e0 -0.25290619 -0.18317325 0 L-histidine EX_cpd00119_e0 -0.0068175 -0.00506825 0 L-proline EX_cpd00129_e0 -0.01102953 0 0 L-malate EX_cpd00130_e0 -0.03649016 -0.79413596 0 D-mannose EX_cpd00138_e0 -0.2540567 -0.05436649 0 Co2 EX_cpd00149_e0 -

Lists of Names of Prokaryotic Candidatus Taxa
NOTIFICATION LIST: CANDIDATUS LIST NO. 1 Oren et al., Int. J. Syst. Evol. Microbiol. DOI 10.1099/ijsem.0.003789 Lists of names of prokaryotic Candidatus taxa Aharon Oren1,*, George M. Garrity2,3, Charles T. Parker3, Maria Chuvochina4 and Martha E. Trujillo5 Abstract We here present annotated lists of names of Candidatus taxa of prokaryotes with ranks between subspecies and class, pro- posed between the mid- 1990s, when the provisional status of Candidatus taxa was first established, and the end of 2018. Where necessary, corrected names are proposed that comply with the current provisions of the International Code of Nomenclature of Prokaryotes and its Orthography appendix. These lists, as well as updated lists of newly published names of Candidatus taxa with additions and corrections to the current lists to be published periodically in the International Journal of Systematic and Evo- lutionary Microbiology, may serve as the basis for the valid publication of the Candidatus names if and when the current propos- als to expand the type material for naming of prokaryotes to also include gene sequences of yet-uncultivated taxa is accepted by the International Committee on Systematics of Prokaryotes. Introduction of the category called Candidatus was first pro- morphology, basis of assignment as Candidatus, habitat, posed by Murray and Schleifer in 1994 [1]. The provisional metabolism and more. However, no such lists have yet been status Candidatus was intended for putative taxa of any rank published in the journal. that could not be described in sufficient details to warrant Currently, the nomenclature of Candidatus taxa is not covered establishment of a novel taxon, usually because of the absence by the rules of the Prokaryotic Code. -
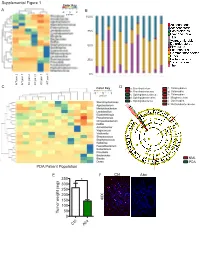
Mental Figure 1 Color Key a -2 0 2 B Z-Score 100%
Supplemental Figure 1 Color Key A -2 0 2 B z-score 100% 75% 50% 25% 0% KC pan 1 WT pan 3 WT KC pan 3 WT pan 2 WT pan 1 WT KC pan 2 C Color Key D a: Brevibacterium f: Chlamydiales b: Brevibacteriaceae g: Chlamydiia -3 0 3 z-score c: Sphingobacteriaceae h: Chlamydiae d: Sphingobacteriales i: Mogibacterium e: Sphingobacteriia j: Oscillospira k: Methylobacteriaceae NML Control Microb.-entrained MΦ PDA PDA Patient Population Control Microb.-entrained MΦ + Myd88i E F Ctrl Abx 350 * 300 250 200 150 40X 100 Tumor weight (mg) 50 0 x Ctrl Ab Supplemental Figure 2 A KC WT B ** * Actinobacteria * ** Bacteroidetes Cyanobacteria Deferribacteres * Firmicutes Proteobacteria % Relative abundance TM7 Others Time(wks) 3 9 13 16 20 24 28 32 36 3 9 13 16 20 24 28 32 36 Alpha Diversity Measure C E 60 KC WT 40 20 B. pseudolongum B. animalis 60 5 KC WT 0 B. adolescentis 40% Rel. abundance 3 9 13 16 20 24 28 32 36 3 9 13 16 20 24 28 32 36 20 Age (weeks) B. pseudolongum B. animalis 5 0 B. adolescentis % Rel. abundance 3 9 13 16 20 24 28 32 36 3 9 13 16 20 24 28 32 36 F Age (weeks) Week 3 Week 9 Week 13 p=0.678 p=0.02 p=0.385 Time(wks) 3 9 24 20 13 16 D 28 32 36 Week 13 KC WT Firmicutes; Ruminococcus Firmicutes; Dehalobacterium Alpha Diversity Measure Firmicutes; Oscillospira Bacteroidates; Odoribacter Axis.2 [12.7%] Actinobacteria; Bifidobacterium Axis.2 [23.8%] Axis.2 [24.7%] Week 16 Bacteroidetes; Bacteroidales Axis.1 [80.8%] Axis.1 [65.4%] Axis.1 [49.6%] Actinobacteria; Bifidobacterium Week 16 Week 20 Week 24 Week 20 p=0.339 p=0.036 p=0.021 Firmicutes; Dehalobacterium