Geological Survey Professional Paper 1022-E
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mount Baker, Washington
WATER-QUALITY EFFECTS ON BAKER LAKE OF RECENT VOLCANIC ACTIVITY AT MOUNT BAKER, WASHINGTON GEOLOGICAL SURVEY PROFESSIONAL PAPER 1022-B Prepared in cooperation with the State of Washington Department of Ecology Water-Quality Effects on Baker Lake of Recent Volcanic Activity at Mount Baker, Washington By G. C. BORTLESON, R. T. WILSON, and B. L. FOXWORTHY VOLCANIC ACTIVITY AT MOUNT BAKER, WASHINGTON GEOLOGICAL SURVEY PROFESSIONAL PAPER 1022-B Prepared in cooperation with the State of Washington Department of Ecology UNITED STATES GOVERNMENT PRINTING OFFICE:1977 UNITED STATES DEPARTMENT OF THE INTERIOR CECIL D. ANDRUS, Secretary GEOLOGICAL SURVEY V. E. McKelvey, Director Library of Congress Cataloging in Publication Data Bortleson, Gilbert Carl, 1940- Water-quality effects on Baker Lake of recent volcanic activity at Mount Baker, Washington. (Volcanic Activity at Mt. Baker) (Geologic Survey Professional Paper 1022-B) Bibliography: p. 30. Supt.ofDocs.no.: I 19.16:1022-6 1. Water quality-Washington (State)--Baker Lake. 2. Volcanism-Washington (State). 3. Baker, Mount, Wash. I. Wilson, Reed T., joint author. II. Foxworthy, Bruce, La Verne, 1925- joint author. III. Washington (State). Dept. of Ecology. IV. Title. V. Series: Volcanic activity at Mount Baker, Washington. VI. Series: United States Geological Survey Professional Paper 1022-B. TD224.W2B67 363.6'1 77-21097 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 Stock Number 024-001-03008-0 CONTENTS Conversion factors _________________________. Ill Quality of surface waters draining to Baker Lake ______B16 Abstract__________________________________. Bl Water in Sherman Crater __________________ 16 Introduction ______________________________. 1 Boulder Creek and other streams _____________. -

Canadian Volcanoes, Based on Recent Seismic Activity; There Are Over 200 Geological Young Volcanic Centres
Volcanoes of Canada 1 V4 C.J. Hickson and M. Ulmi, Jan. 3, 2006 • Global Volcanism and Plate tectonics Where do volcanoes occur? Driving forces • Volcano chemistry and eruption types • Volcanic Hazards Pyroclastic flows and surges Lava flows Ash fall (tephra) Lahars/Debris Flows Debris Avalanches Volcanic Gases • Anatomy of an Eruption – Mt. St. Helens • Volcanoes of Canada Stikine volcanic belt Presentation Outline Anahim volcanic belt Wells Gray – Clearwater volcanic field 2 Garibaldi volcanic belt • USA volcanoes – Cascade Magmatic Arc V4 Volcanoes in Our Backyard Global Volcanism and Plate tectonics In Canada, British Columbia and Yukon are the host to a vast wealth of volcanic 3 landforms. V4 How many active volcanoes are there on Earth? • Erupting now about 20 • Each year 50-70 • Each decade about 160 • Historical eruptions about 550 Global Volcanism and Plate tectonics • Holocene eruptions (last 10,000 years) about 1500 Although none of Canada’s volcanoes are erupting now, they have been active as recently as a couple of 4 hundred years ago. V4 The Earth’s Beginning Global Volcanism and Plate tectonics 5 V4 The Earth’s Beginning These global forces have created, mountain Global Volcanism and Plate tectonics ranges, continents and oceans. 6 V4 continental crust ic ocean crust mantle Where do volcanoes occur? Global Volcanism and Plate tectonics 7 V4 Driving Forces: Moving Plates Global Volcanism and Plate tectonics 8 V4 Driving Forces: Subduction Global Volcanism and Plate tectonics 9 V4 Driving Forces: Hot Spots Global Volcanism and Plate tectonics 10 V4 Driving Forces: Rifting Global Volcanism and Plate tectonics Ocean plates moving apart create new crust. -

Geological Survey Professional Paper 1022-C
GEOLOGICAL SURVEY PROFESSIONAL PAPER 1022-C POSTGLACIAL VOLCANIC DEPOSITS ATMOUNTBAKER, WASHINGTON AND POTENTIAL HAZARDS FROM FUTURE ERUPTIONS FRONTISPIECE.- East side of Mount Baker. Boulder Creek, in the center of the photograph, flows into Baker Lake. Postglacial Volcanic Deposits at Mount Baker, Washington, and Potential Hazards from Future Eruptions By JACK H. HYDE and DWIGHT R CRANDELL VOLCANIC ACTIVITY AT MOUNT BAKER, WASHINGTON G E 0 L 0 G I CAL SURVEY P R 0 FE S S I 0 N A L PAPER 1 0 2 2 -C An assessment of potential hazards at Mount Baker is based on the volcano's eruptive behavior during the last 10, 000 years UNITED STATES GOVERNMENT PRINTING OFFICE, \Y ASHINGTON : 1978 UNITED STATES DEPARTMENT OF THE INTERIOR CECIL D. ANDRUS, Secretary GEOLOGICAL SURVEY H. William Menard, Director First Printing 1978 Second Printing 1981 Library of Congress Cataloging in Publication Data Hyde, Jack H. J'()stglacial volcanic deposits at Mount Baker. Washington, and potential hazards from future eruptions. (Volcanic activity at Mount Baker, Washington) Geological Survey Professional Paper 1022-C Bibliography: p. 16 Supt. of Docs. No.: I 19.16:1022-C I. Volcanic ash, tuff, etc.-Washington (State)-Baker, Mount. 2. Volcanism-Washington (State)-Baker, Mount. 3. Volcanic activity prediction. I. Crandell, Dwight Raymond, 1923- joint author. II. Title: Postglacial volcanic deposits at Mount Baker, Washington, and potential hazards from future eruptions. III. Series: IV. Series: United States Geological Survey Professional Paper 1022-C. QE46I.H976 55 1.2'2'09797 77-5891 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. -

Ore Bin / Oregon Geology Magazine / Journal
VOLUME 40, No. 8 AUGUST 1978 STATE OF OREGON DEPARTMENT OF GEOLOGY AND MINERAL INDUSTRIES The Ore Bin Published Monthly by STATE OF OREGON DEPARTMENT OF GEOLOGY AND MINERAL INDUSTRIES Head Office: 1069 State Office Bldg .• Port l and 9720 1 Telephone: [503] 229-5580 FI ElD OFFI CES 2033 First Street 521 N.£. "E" Street Baker 97814 Grants Pass 97526 MINED lAND RECLAMATION DIVISION 1129 S.E . Santiam Road Albany 97321 Subscription Rates 1 year, $3.00; 3 years, $8.00 Available back issues: $.25 at counter. S.50 ~iled Second class postage paid at Portland. Oregon GOVERNING BOARD Leeanne MacCo 11. Portland Robert W. Doty. Talent John L. Schwabe. Portland STATE GEOLOGIST Donald A. Hull GEOLOGISTS IN CHARGE OF FIELD OFFICES Howard C. Brooks. Baker Len Ramp. Grants Pass EDITOR Beverly F. Vogt Pe .... lsslon to reprint Information contained herein is granted. Credit • given the State of Oregon Department of Geology and Mineral Industries will be appreciated. State of Oregon Oepa rtment of Geo logy The ORE BIN and Mineral Industries Volume 40, No.8 1069 State Office Bldg. Port 1and, Oregon 97201 August 1978 MOUNT BAKER'S CHANGING FUMAROLES Eugene P. Kiver Department of Geology Eastern Washington University Introduction On March 10, 1975, a dark fume cloud rising a few hundred meters above the subsummit crater (Sherman Crater) of 3,286-m-high (10,781-ft-high) Mount Baker (Figures 1 and 2) in the North Cascade Mountains of Washington generated considerable concern among scientists, government officials, and local residents (Frank and others, 1977; Kiver, 1975; Malone and Frank, 1975; 121°30' and Rosenfel d and .~--:-:-c="",,~----:;"-------------'~ Sch 1i c ke r , 1976 ) . -

Surficial Extent and Conceptual Model of Hydrothermal System at Mount Rainier, Washington
Journal of Volcanology and Geothermal Research 65 ( 1995) 5 l-80 Surficial extent and conceptual model of hydrothermal system at Mount Rainier, Washington David Frank U.S.Environmental Protection Agency, 1200 Sixth Avenue, ES-098 Seattle, WA 98101, USA Received 8 January 1992; revised version accepted 10 February 1993* Abstract A once massive hydrothermal system was disgorged from the summit of Mount Rainier in a highly destructive manner about 5000 years ago. Today, hydrothermal processes are depositing clayey alteration products that have the potential to reset the stage for similar events in the future. Areas of active hydrothermal alteration occur in three representative settings: ( 1) An extensive area (greater than 12,000 m*) of heated ground and slightly acidic boiling-point fumaroles at 76-82°C at East and West Craters on the volcano’s summit, where alteration products include smectite, halloysite and disordered kaolinite, cristobalite, tridymite, opal, alunite, gibbsite, and calcite. (2) A small area (less than 500 m’) of heated ground and sub-boiling-point fumaroles at 55-60°C on the upper flank at Disappointment Cleaver with smectite alteration and chalcedony, tridymite, and opal-A encrustations. Similar areas probably occur at Willis Wall, Sunset Amphitheater, and the South Tahoma and Kautz headwalls. (3) Sulfate- and carbon dioxide-enriched thermal springs at 9-24°C on the lower flank of the volcano in valley walls beside the Winthrop and Paradise Glaciers, where calcite, opal-A, and gypsum are being deposited. In addition, chloride- and carbon dioxide-enriched thermal springs issue from thin sediments that overlie Tertiary rocks at, or somewhat beyond, the base of the volcanic edifice in valley bottoms of the Nisqually and Ohanapecosh Rivers. -
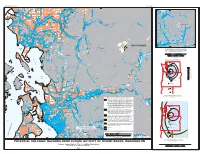
Mt. Baker Volcanic Hazards
U.S. DEPARTMENT OF THE INTERIOR OPEN-FILE REPORT U.S. GEOLOGICAL SURVEY 122 0 95-492 122 0 Sumas Lynden Glacier N or Nooksack th Birch F o Deming Bay rk Everson River Nooksack Van Zandt MOUNT BAKER Nooksack River Ferndale Deming M i d d l e Concrete F o r k 0 Sedro Woolley 48 30' MOUNT BAKER Rockport Nooksack River Lummi Lateral Blast Hazard Zone. Area that could be affected by a lateral Bay blast similar in size to the May 18, 1980 lateral blast event at Mount St. Helens. During any given lateral blast the entire area around the volcano (360 degrees) would not be affected, but rather a sector most likely between 90 and 180 degrees. SCALE 1: 250 000 Lake 5 0 5 10 15 MILES Whatcom 5 0 5 10 15 20 KILOMETERS Contour interval 400 meters Bellingham Bay Baker Lake Total Cascades Tephra Hazards Mount Baker Glacier Peak 1 % Mount Rainier 0.2 % Mount St. Helens Mount Adams 0.1 % 0.02 % Mount Hood 0.01 % Samish Mount Jefferson Bay < 0.01 % Three Sisters Lake Newberry Volcano Shannon Crater Lake Edison Medicine Lake Mount Shasta Padilla Bay Skagit River Lassen Peak Sedro Woolley Hamilton Annual probability of the deposition of 10 centimeters (4 inches) or more of tephra from any of the major Cascade 480 30' Volcanoes. Explanation of Flowage Hazard Zones Inundation zone for Case M debris flows: Area that could be affected by cohesive Burlington debris flows that originate as large debris avalanches of hydrothermally altered rock from the volcanic edifice. -

LOCATION of VOLCANOES in BRITISH COLUMBIA Source of Volcanic Eruption Hazards to the Lower Mainland of British Columbia
LOCATION OF VOLCANOES IN BRITISH COLUMBIA Source of Volcanic Eruption Hazards to the Lower Mainland of British Columbia RELIEF MAP OF BRITISH COLUMBIA VOLCANOES IN BC (from North to South) Ruby Mountain Heart Peaks Level Mountain Edziza Spectrum Range Hoodoo Mountain Yseax River Cone Iskut-Unuk River Crow Lagoon Mulbanke Sound Satah Mountain Mt. Garibaldi Volcano located in British Columbia Mt. Garibaldi (Volcano) located in Washington Nazko Eruption History: last dated 10,000 years ago Eruption History: 1792, 1843 -65 , 1870, 1880 "The volcanic rocks are divided into five groups with diverse types of volcanoes Wells Gray "Historical Activity Historical activity at Mount Baker includes several and tectonic settings. In southern British Columbia, the Pemberton and Garibaldi Siverthrone explosions during the mid-19th century, which were witnessed from the volcanic belts and the Chilcotin Group plateau are related to the subduction of Bridge River Bellingham area, and since the late 1950s, numerous small- volume debris the Juan de Fuca and Explorer plates beneath the North American continent. The Meager avalanches. In 1975, increased fumarolic activity in the Sherman Crater area caused concern that an eruption might be imminent. Additional monitoring Anahim Volcanic Belt trends easterly across central British Columbia and is Garibaldi probably related to a mantle hot spot. The Stikine Volcanic Belt forms a broad equipment was installed and several geophysical surveys were conducted to try to zone of volcanoes in northwestern British Columbia and the southern Yukon. These Baker (Washington) detect the movement of magma. The level of Baker Lake was lowered and people volcanoes are probably related to shear along the Queen Charolette transform were restricted from the area due to concerns that an eruption- induced debris fault to the west. -

Characterizing Surface Deformation from 1981 to 2007 on Mount Baker Volcano, Washington
Western Washington University Western CEDAR WWU Graduate School Collection WWU Graduate and Undergraduate Scholarship Spring 2008 Characterizing Surface Deformation from 1981 to 2007 on Mount Baker Volcano, Washington Brendan E. Hodge Western Washington University, [email protected] Follow this and additional works at: https://cedar.wwu.edu/wwuet Part of the Geology Commons Recommended Citation Hodge, Brendan E., "Characterizing Surface Deformation from 1981 to 2007 on Mount Baker Volcano, Washington" (2008). WWU Graduate School Collection. 666. https://cedar.wwu.edu/wwuet/666 This Masters Thesis is brought to you for free and open access by the WWU Graduate and Undergraduate Scholarship at Western CEDAR. It has been accepted for inclusion in WWU Graduate School Collection by an authorized administrator of Western CEDAR. For more information, please contact [email protected]. CHARACTERIZING SURFACE DEFORMATION FROM 1981 TO 2007 ON MOUNT BAKER VOLCANO, WASHINGTON BY BRENDAN E. HODGE Accepted in Partial Completion of the Requirements for the Degree Master of Science Moheb A. Ghali, Dean of the Graduate School Dr. Pete Sfelling MASTER’S THESIS In presenting this thesis in partial fulfillment of the requirements for a master’s degree at Western Washington University, I agree that the Library shall make its copies freely available for inspection. I further agree that copying of this thesis in whole or in part is allowable only for scholarly purposes. It is understood, however, that any copying or publication of this thesis for commercial purposes, or for financial gain, shall not be allowed without my written permission. MASTER’S THESIS In presenting this thesis in partial fulfillment of the requirements for a master’s degree at Western Washington University, I grant to Western Washington University the non-exclusive royalty-free right to archive, reproduce, distribute, and display the thesis in any and all forms, including electronic format, via any digital library mechanisms maintained by WWU. -

USGS Fact Sheet 165-97
U.S. GEOLOGICAL SURVEY—REDUCING THE RISK FROM VOLCANO HAZARDS Living with Volcanic Risk in the Cascades he Cascade Range of the Pacific TNorthwest has more than a dozen potentially active volcanoes. Cascade volcanoes tend to erupt explosively, and on average two eruptions occur per century—the most recent were at Mount St. Helens, Washington (1980–86), and Lassen Peak, Cali- fornia (1914–17). To help protect the Pacific Northwest’s rapidly expand- ing population, USGS scientists at the Cascades Volcano Observatory in Vancouver, Washington, monitor and assess the hazards posed by the region’s volcanoes. On May 18, 1980, after 2 months of earthquakes and minor eruptions, Mount St. The more than 1 million residents of the Seattle-Tacoma, Washing- Helens, Washington, exploded in one of the ton, area live in the shadow of 14,411-foot-high Mount Rainier, the most devastating volcanic eruptions of the tallest volcano in the United States outside of Alaska. Several communities near the volcano, built on the 20th century. Although less than 0.1 cubic deposits of giant lahars of volcanic ash and debris that are less than 1,200 years old, are at risk from similar future lahars. Inset photograph shows a U.S. Geological Survey (USGS) scientist monitoring the volcano for mile of molten rock (magma) was erupted, 57 signs of subtle ground movement that might lead to an eruption or landslide. Photos by Lyn Topinka, USGS. people died, and damage exceeded $1 billion. Fortunately, most people in the area were able (pyroclastic flows), lava flows, and landslides years ago, an especially powerful series of to evacuate safely before the eruption because can devastate areas 10 or more miles away; eruptions deposited volcanic ash at least as far public officials had been alerted to the danger and huge mudflows of volcanic ash and debris, away as Wyoming. -

Glacial and Geothermal Dynamics in Sherman Crater, Mount Baker, Washington
Western Washington University Western CEDAR WWU Graduate School Collection WWU Graduate and Undergraduate Scholarship 2011 Glacial and geothermal dynamics in Sherman Crater, Mount Baker, Washington Melissa Park Western Washington University Follow this and additional works at: https://cedar.wwu.edu/wwuet Part of the Geology Commons Recommended Citation Park, Melissa, "Glacial and geothermal dynamics in Sherman Crater, Mount Baker, Washington" (2011). WWU Graduate School Collection. 139. https://cedar.wwu.edu/wwuet/139 This Masters Thesis is brought to you for free and open access by the WWU Graduate and Undergraduate Scholarship at Western CEDAR. It has been accepted for inclusion in WWU Graduate School Collection by an authorized administrator of Western CEDAR. For more information, please contact [email protected]. Glacial and geothermal dynamics in Sherman Crater, Mount Baker, Washington By Melissa Park Accepted in Partial Completion Of the Requirements for the Degree Master of Science ________________________ Moheb A. Ghali, Dean of the Graduate School ADVISORY COMMITTEE ________________________ Chair, Dr. Douglas H. Clark ________________________ Dr. Jackie Caplan-Auerbach ________________________ Dr. Bernard Housen MASTER’S THESIS In presenting this thesis in partial fulfillment of the requirements for a master’s degree at Western Washington University, I grant to Western Washington University the non‐exclusive royalty‐free right to archive, reproduce, distribute, and display the thesis in any and all forms, including electronic format, via any digital library mechanisms maintained by WWU. I represent and warrant this is my original work, and does not infringe or violate any rights of others. I warrant that I have obtained written permissions from the owner of any third party copyrighted material included in these files. -

80, and 1975 Steam Emission Glacier Peak Ash 1+? Before 1800 (1750?) Tephra Between 1830 and Mount Rainier Ash, Lava 1? 1854 Ash, Lava, Mount St
Whatcom County Natural Hazards Mitigation Plan A MULTI-HAZARD, MULTI-JURISDICTIONAL PLAN DEVELOPED FOR THE BENEFIT OF ALL CITIZENS AND GOVERNMENTAL JURISDICTIONS WITHIN WHATCOM COUNTY Prepared by: Whatcom County Division of Emergency Management June 1, 2015 Whatcom County SECTION 2: HAZARD SUMMARIES Natural Hazards Mitigation Plan VOLCANOES A. DEFINITIONS Blast Zone The area immediately surrounding a volcano, up to several tens of kilometers, that is destroyed by a volcano’s blast. Lava Flow A stream of molten rock that pours or oozes from an erupting vent. Lahar A mudflow or debris flow that originates from the slope of a volcano; pyroclastic flows can generate lahars by rapidly melting snow and ice. Pyroclastic Flows High-density mixtures of hot, dry rock fragments and hot gases that move away from the vent that erupted them at high speeds. Tephra General term for fragments of volcanic material, regardless of size, that are blasted into the air by explosions or carried up upward by hot gases in eruption columns or lava fountains. Volcano A vent in the earth’s crust through which magma (molten rock), rock fragments, associated gases, and ashes erupt, and also the cone built by effusive and explosive eruptions. B. BACKGROUND INFORMATION The Cascade Range (Cascades) extends more than 1,000 miles, forming an arc-shaped band extending from Southern B.C. to Northern California. The Cascades roughly parallels the Pacific coastline, and at least 17 major volcanic centers. Whatcom County’s eastern boundary follows the crest of the Cascade Range. The central and southern Cascades are made up of a band of thousands of much older, smaller, short- lived volcanoes that have built a platform of lava and volcanic debris. -

Mount Baker – Studying the Active Volcano in Our Backyard
the active involcano our backyard - Baker Mount Baker – studying the Mount Mount active volcano in our backyard mbvrc.wwu.edu mbvrc.wwu.edu Dave Tucker Mount Baker Volcano Research Center Geology Department Western Washington University 1 Acknowledgements: my colleagues in the field Kevin Scott, USGS Dave Lewis, Wes Hildreth, USGS MBHS the active involcano our backyard - Baker Mount Mount mbvrc.wwu.edu mbvrc.wwu.edu Jon Riedel, NCNP 2 Melissa Park, WWU Kristin Hill, WWU Recent research gravity studies Dr. Bob Watters U Nevada- Reno rock stability Brendan Hodge, WWU the active involcano our backyard - volcano deformation Nikki Moore, WWU Baker basalt chemistry Troy Baggerman, WWU Mount Mount andesite chemistry mbvrc.wwu.edu mbvrc.wwu.edu Dr. J. Caplan-Auerbach, WWU, seismology Melissa Park, WWU Sarah Polster, USGS Sherman Crater Sherman Crater magnetic 3 morphology survey 4 mbvrc.wwu.edu Mount Baker- the active volcano in our backyard Field work at Field work Baker 5 mbvrc.wwu.edu Mount Baker- the active volcano in our backyard Getting there Getting 6 mbvrc.wwu.edu Mount Baker- the active volcano in our backyard 7 mbvrc.wwu.edu Mount Baker- the active volcano in our backyard Tephra sampling Tephra Volcanic ash layers at Baker ash layers Volcanic Gravimetry Geodesy- GPS the active involcano our backyard - Baker Mount Mount dendrochronology mbvrc.wwu.edu mbvrc.wwu.edu 8 9 mbvrc.wwu.edu Mount Baker- the active volcano in our backyard Stream Stream gaging seismometry Fumarole gas collection gas Fumarole 10 mbvrc.wwu.edu Mount Baker- the active