Simplified HIV Testing and Treatment in China: Analysis of Mortality Rates Before and After a Structural Intervention
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Investigation and Analysis of Genetic Diversity of Diospyros Germplasms Using Scot Molecular Markers in Guangxi
RESEARCH ARTICLE Investigation and Analysis of Genetic Diversity of Diospyros Germplasms Using SCoT Molecular Markers in Guangxi Libao Deng1,3☯, Qingzhi Liang2☯, Xinhua He1,4*, Cong Luo1, Hu Chen1, Zhenshi Qin5 1 Agricultural College of Guangxi University, Nanning 530004, China, 2 National Field Genebank for Tropical Fruit, South Subtropical Crops Research Institutes, Chinese Academy of Tropical Agricultural Sciences, Zhanjiang 524091, China, 3 Administration Committee of Guangxi Baise National Agricultural Science and Technology Zone, Baise 533612, China, 4 Guangxi Crop Genetic Improvement and Biotechnology Laboratory, Nanning 530007, China, 5 Experiment Station of Guangxi Subtropical Crop Research Institute, Chongzuo 532415, China ☯ These authors contributed equally to this work. * [email protected] Abstract OPEN ACCESS Citation: Deng L, Liang Q, He X, Luo C, Chen H, Qin Background Z (2015) Investigation and Analysis of Genetic Diversity of Diospyros Germplasms Using SCoT Knowledge about genetic diversity and relationships among germplasms could be an Molecular Markers in Guangxi. PLoS ONE 10(8): invaluable aid in diospyros improvement strategies. e0136510. doi:10.1371/journal.pone.0136510 Editor: Swarup Kumar Parida, National Institute of Methods Plant Genome Research (NIPGR), INDIA This study was designed to analyze the genetic diversity and relationship of local and natu- Received: January 1, 2015 ral varieties in Guangxi Zhuang Autonomous Region of China using start codon targeted Accepted: August 5, 2015 polymorphism (SCoT) markers. The accessions of 95 diospyros germplasms belonging to Published: August 28, 2015 four species Diospyros kaki Thunb, D. oleifera Cheng, D. kaki var. silverstris Mak, and D. Copyright: © 2015 Deng et al. This is an open lotus Linn were collected from different eco-climatic zones in Guangxi and were analyzed access article distributed under the terms of the using SCoT markers. -

The Globalization of Chinese Food ANTHROPOLOGY of ASIA SERIES Series Editor: Grant Evans, University Ofhong Kong
The Globalization of Chinese Food ANTHROPOLOGY OF ASIA SERIES Series Editor: Grant Evans, University ofHong Kong Asia today is one ofthe most dynamic regions ofthe world. The previously predominant image of 'timeless peasants' has given way to the image of fast-paced business people, mass consumerism and high-rise urban conglomerations. Yet much discourse remains entrenched in the polarities of 'East vs. West', 'Tradition vs. Change'. This series hopes to provide a forum for anthropological studies which break with such polarities. It will publish titles dealing with cosmopolitanism, cultural identity, representa tions, arts and performance. The complexities of urban Asia, its elites, its political rituals, and its families will also be explored. Dangerous Blood, Refined Souls Death Rituals among the Chinese in Singapore Tong Chee Kiong Folk Art Potters ofJapan Beyond an Anthropology of Aesthetics Brian Moeran Hong Kong The Anthropology of a Chinese Metropolis Edited by Grant Evans and Maria Tam Anthropology and Colonialism in Asia and Oceania Jan van Bremen and Akitoshi Shimizu Japanese Bosses, Chinese Workers Power and Control in a Hong Kong Megastore WOng Heung wah The Legend ofthe Golden Boat Regulation, Trade and Traders in the Borderlands of Laos, Thailand, China and Burma Andrew walker Cultural Crisis and Social Memory Politics of the Past in the Thai World Edited by Shigeharu Tanabe and Charles R Keyes The Globalization of Chinese Food Edited by David Y. H. Wu and Sidney C. H. Cheung The Globalization of Chinese Food Edited by David Y. H. Wu and Sidney C. H. Cheung UNIVERSITY OF HAWAI'I PRESS HONOLULU Editorial Matter © 2002 David Y. -

Anisotropic Patterns of Liver Cancer Prevalence in Guangxi in Southwest China: Is Local Climate a Contributing Factor?
DOI:http://dx.doi.org/10.7314/APJCP.2015.16.8.3579 Anisotropic Patterns of Liver Cancer Prevalence in Guangxi in Southwest China: Is Local Climate a Contributing Factor? RESEARCH ARTICLE Anisotropic Patterns of Liver Cancer Prevalence in Guangxi in Southwest China: Is Local Climate a Contributing Factor? Wei Deng1&, Long Long2&*, Xian-Yan Tang3, Tian-Ren Huang1, Ji-Lin Li1, Min- Hua Rong1, Ke-Zhi Li1, Hai-Zhou Liu1 Abstract Geographic information system (GIS) technology has useful applications for epidemiology, enabling the detection of spatial patterns of disease dispersion and locating geographic areas at increased risk. In this study, we applied GIS technology to characterize the spatial pattern of mortality due to liver cancer in the autonomous region of Guangxi Zhuang in southwest China. A database with liver cancer mortality data for 1971-1973, 1990-1992, and 2004-2005, including geographic locations and climate conditions, was constructed, and the appropriate associations were investigated. It was found that the regions with the highest mortality rates were central Guangxi with Guigang City at the center, and southwest Guangxi centered in Fusui County. Regions with the lowest mortality rates were eastern Guangxi with Pingnan County at the center, and northern Guangxi centered in Sanjiang and Rongshui counties. Regarding climate conditions, in the 1990s the mortality rate of liver cancer positively correlated with average temperature and average minimum temperature, and negatively correlated with average precipitation. In 2004 through 2005, mortality due to liver cancer positively correlated with the average minimum temperature. Regions of high mortality had lower average humidity and higher average barometric pressure than did regions of low mortality. -
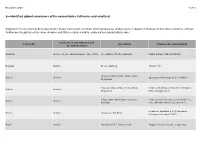
2—Identified Global Consumers of Tin Concentrates (Refineries and Smelters)
November 2020 1 of 12 2—Identified global consumers of tin concentrates (refineries and smelters) Explanation: This list contains global facilities known to be able to process tin concentrate. Additional small-scale smelters may be in operation in Indonesia, but their status could not be confirmed. Facilities were thought to be active unless otherwise noted. Data records are sorted by country and then alphabetically by owner. FACILITY TYPE AND STATUS COUNTRY LOCATION OPERATOR / OWNERSHIP (IF APPLICABLE) Australia Smelter (on care and maintenance since 2012) Greenbushes, Western Australia Global Advanced Metals Pty Ltd. Belgium Smelter Beerse, Antwerp Aurubis AG Huajara Industrial Park, Oruro, Oruro Bolivia Smelter Operaciones Metalúrgicas S.A. (OMSA) Department Vinto smelting complex, Vinto, Oruro Empresa Metalúrgica Vinto S.A. (Compania Bolivia Smelter Department Minera Colquira S.A.) Coopersanta, Bom Futuro, Ariquemes, Cooperativa de Garimperiros de Santa Cruz Brazil Smelter Rondônia Ltda. [Meridian Mineração Jaburi S.A.] Estanho de Rondônia S.A. [Companhia Brazil Smelter Ariquemes, Rondônia Siderúrgica Nacional (CSN)] Brazil Smelter São João del Rei, Minas Gerais Magnu's Minerais Metais e Ligas Ltda. November 2020 2 of 12 2—Identified global consumers of tin concentrates (refineries and smelters) Explanation: This list contains global facilities known to be able to process tin concentrate. Additional small-scale smelters may be in operation in Indonesia, but their status could not be confirmed. Facilities were thought to be active unless otherwise noted. Data records are sorted by country and then alphabetically by owner. FACILITY TYPE AND STATUS COUNTRY LOCATION OPERATOR / OWNERSHIP (IF APPLICABLE) Brazil Smelter Ariquemes, Rondônia Melt Metais e Ligas S.A. -

50391-001: Demonstration of Guangxi Elderly Care and Health Care
Resettlement Plan May 2019 People’s Republic of China: Demonstration of Guangxi Elderly Care and Health Care Integration and Public–Private Participation Project Hezhou No. 2 Nursing Home for Disabled Elderly Prepared by Guangxi Zhuang Autonomous Region for the Asian Development Bank. CURRENCY EQUIVALENTS (as of 29 April 2019) Currency unit – yuan (Symbol) CNY1.00 = $6.7297 $1.00 = CNY0.1486 ABBREVIATIONS ADB – Asian Development Bank AFs – affected families APs – affected persons DI – design institute DMS – detailed measurement survey EA – executive agency HLRB – Hezhou Land Resources Administration Bureau HMG – Hezhou Municipal Government HPMO – Hezhou project management office FSR – feasibility study report GDP – gross domestic product GZAR – Guangxi Zhuang Autonomous Region HHs – households IA – implementing agency IMA – independent monitoring agency LA – land acquisition LAR – land acquisition and resettlement LRB – land resources bureau M&E – monitoring and evaluation MLG – minimum living guarantee OP – operation procedures PLG – project leading group PMO – project management office PPTA – project preparation technical assistance PRC – People’s Republic of China RC – residents' committee RIB – resettlement information brochure RO – resettlement office ROW – right-of-way RAP – resettlement plan S&T – science and technology TOR – terms of reference WF – women’s federation km2 – square kilometer mu – A mu is a Chinese unit of measurement (1 mu = 666.667 square meters). NOTE In this report, "$" refers to United States dollars. This resettlement plan is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, Management, or staff, and may be preliminary in nature. Your attention is directed to the “terms of use” section of this website. -

Red History Thriving Along Highway Guangdong Province
CHINA DAILY | HONG KONG EDITION Monday, May 3, 2021 | 11 TRAVEL PATHWAYS TO PROGRESS Expressway leads to a big discovery: Prosperity By ZHANG LI in Nanning [email protected] Mo Shuifeng moves swiftly and expertly between the vegetables she grows for guests in her garden in Hetang village, Zhongshan county, Hezhou of Guangxi Zhuang autono- mous region. She feels she is dreaming. Just five years ago she was living in poverty because of the financial burden of her children’s school tuition. With limited transport and a lack of arable land for farming, Hetang had been one of the poorest areas of the county. In 2015, severe poverty affected more than 33 percent of the population. But the village had a secret asset that had never been tapped: It Pupils of the Zhaojin Beiliang Red Army Primary School in Zhaojin town, Tongchuan, Shaanxi province, learn the history of the Communist Party of China. boasts 16 kilometers of picturesque PROVIDED TO CHINA DAILY karst landscape that few people have ever heard about. In 2017, an expressway linked Baotou in the Inner Mongolia autonomous region with Maoming, Red history thriving along highway Guangdong province. The express- way passes Guilin, Wuzhou and Hezhou in Guangxi. Located just 11 km from the Rich experiences Baotou-Maoming tion,” he wrote in his diary. expressway entrance, the scenic Expressway Tongdao Dong autonomous landscape of Hetang village could await those who county of Huaihua, Hunan prov- not stay hidden and was soon dis- want to get a feel Baotou ince, along the expressway, was the covered by the world. -

Conservation Recommendations for Oryza Rufipogon Griff. in China
www.nature.com/scientificreports OPEN Conservation recommendations for Oryza rufpogon Grif. in China based on genetic diversity analysis Junrui Wang1,6, Jinxia Shi2,6, Sha Liu1, Xiping Sun3, Juan Huang1,4, Weihua Qiao1,5, Yunlian Cheng1, Lifang Zhang1, Xiaoming Zheng1,5* & Qingwen Yang1,5* Over the past 30 years, human disturbance and habitat fragmentation have severely endangered the survival of common wild rice (Oryza rufpogon Grif.) in China. A better understanding of the genetic structure of O. rufpogon populations will therefore be useful for the development of conservation strategies. We examined the diversity and genetic structure of natural O. rufpogon populations at the national, provincial, and local levels using simple sequence repeat (SSR) markers. Twenty representative populations from sites across China showed high levels of genetic variability, and approximately 44% of the total genetic variation was among populations. At the local level, we studied fourteen populations in Guangxi Province and four populations in Jiangxi Province. Populations from similar ecosystems showed less genetic diferentiation, and local environmental conditions rather than geographic distance appeared to have infuenced gene fow during population genetic evolution. We identifed a triangular area, including northern Hainan, southern Guangdong, and southwestern Guangxi, as the genetic diversity center of O. rufpogon in China, and we proposed that this area should be given priority during the development of ex situ and in situ conservation strategies. Populations from less common ecosystem types should also be given priority for in situ conservation. Common wild rice (Oryza rufpogon Grif.) is the putative progenitor of Asian cultivated rice, one of the most important food crops in the world. -

4.5 Ethnic Minority Groups
IPP319 Public Disclosure Authorized The Guiyang-Guangzhou New Railway Construction (GGR) Social Assessment & Ethnic Minority Development Plan Public Disclosure Authorized Public Disclosure Authorized Foreign I&T Introduction Center of MOR, China West China Development Research Center of The Central University of Nationalities Public Disclosure Authorized August 30, 2008 1 3URMHFW7LWOH Social Assessment & Ethnic Minority Development Plan for the Guiyang-Guangzhou New Railway Construction 3URMHFW8QGHUWDNHUV Professor/Dr. Zhang Haiyang (Han) Director of the West China Development Research Center Associate Professor/Dr. Jia Zhongyi (Miao/Mhong) Deputy Director of the WCDRC The Central University of Nationalities, Beijing, 100081 China [email protected]; [email protected] 7DVNIRUFH0HPEHU Chen weifan, female, Hui, graduate students of CUN Zhong wenhong, male, She, graduate student of CUN Shen Jie, femal, Han, graduate student of CUN Feng An, male, Buyi, graduate student of CUN Wu Huicheng, male, Zhuang, graduate student of CUN 'UDIWHUV Jia Zhongyi, Zhang Haiyang, Shen Jie, Chen weifan, Zhong wenhong, Feng An 7UDQVODWRUVZhang Haiyang, Saihan, Liu Liu, Chai Ling , Liang Hongling, Yan Ying, Liang Xining 2 Table of Contents Abstract......................................................................................................................................................................5 Chpt.1 GGR Content & Regional Development Survey............................................................................................9 1.1 Background -

Microsoft to Create 2,000 New Jobs Reforms To
14 | Wednesday, December 16, 2020 HONG KONG EDITION | CHINA DAILY BUSINESS Delisting Microsoft to create 2,000 new jobs reforms to Tech behemoth Microsoft to increase customer base streamline during the pandemic. Crozier said sees huge growth Microsoft has booked more Chinese opportunities in customers, which are chiefly from capital the gaming and entertainment China’s digital industry, to embrace its technolo- gies to go global, as social distancing markets transformation made more people embrace digital entertainment. By ZHOU MO By MA SI Meanwhile, multinational corpo- in Shenzhen, Guangdong [email protected] rations are also placing greater [email protected] emphasis on the China market Technology heavyweight Micro- where economic activities rebound- The revised delisting rules for soft Corp plans to create nearly ed significantly and Microsoft’s listed companies will improve 2,000 new jobs in China in the next technologies can help them better the ecosystem of China’s capital 18 months as it sees strong opportu- innovate in China, and scale their markets and pave the way for the nities in accelerated digital transfor- solutions to global markets, he said. country’s high-quality economic mation in the world’s second-largest Despite geopolitical uncertain- transformation, analysts said. economy. ties, Crozier said many companies The Shanghai Stock Exchange The move is part of Microsoft’s now look at China as a growth plat- and the Shenzhen Stock long-term commitment to the China form for not just this year but next Exchange published draft docu- market, a strategy that has never year and beyond. China is also an ments for consultation late on been disrupted by the COVID-19 pan- important innovation platform Monday, with proposed reforms demic, said Alain Crozier, chairman where the products and technolo- of delisting criteria, channels and CEO of Microsoft Greater China. -

Open Dissertation-XIANG.Pdf
The Pennsylvania State University The Graduate School The College of the Liberal Arts LAND, CHURCH, AND POWER: FRENCH CATHOLIC MISSION IN GUANGZHOU, 1840-1930 A Dissertation in History by Hongyan Xiang 2014 Hongyan Xiang Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy August 2014 ii The dissertation of Hongyan Xiang was reviewed and approved* by the following: Ronnie Hsia Edwin Earle Sparks Professor of History Dissertation Advisor Chair of Committee David G. Atwill Associate Professor of History and Asian Studies Kate Merkel-Hess Assistant Professor of History and Asian Studies Anouk Patel-Campillo Assistant Professor of Rural Sociology Michael Kulikowski Professor of History and Classics and Ancient Mediterranean Studies Head, Department of History *Signatures are on file in the Graduate School iii Abstract This is a study of the economic and financial history of the Paris Foreign Missions Society (Société des Missions Étrangères de Paris) in the southern Chinese province of Guangdong (formerly known as Canton) from the late nineteenth to the early twentieth century. It examines how missionaries acquired and utilized local properties, demonstrating how property acquisitions provided a testing ground for Sino-Western relations. While historians have typically focused on the ways that missionaries affected Chinese populations and policies, I instead argue that living and attempting to gain influence in Guangdong altered missionaries’ tactics and strategies in ways that had far-reaching consequences. The government of China (which over the course of my study changed from an empire to a republic) consistently attempted to restrict foreign missions’ right to purchase Chinese properties. -

World Bank Document
E2008 V3 China Public Disclosure Authorized Guiyang-Gangzhou Railway Project Environmental Impact Assessment Executive Summary October, 2008 Public Disclosure Authorized Public Disclosure Authorized Public Disclosure Authorized Guiyang-Guangzhou Railway Project Environmental Impact Assessment Executive Summary INTRODUCTION Background This document summarizes the environment impact assessment of the Guiyang-Guangzhou Railway Project in China, highlighting the main issues and conclusions of the environment impact assessment and environment management plan of the project. According to both Chinese Environmental Assessment laws and regulations and the World Bank’s Operational Policy 4.01 Environmental Assessment, the proposed project is Category A for environmental assessment purposes, due to the scale and significance of potential environmental and social impacts and the sensitivity of the project areas. Therefore, a full environmental assessment report was required. The Ministry of Railways (MOR) retained China Railway Second Survey and Design Institute (SSDI) and China Railway Fourth Survey and Design Institute (FSDI) for EA preparation. Both institutes hold Class A environmental impact assessment accreditation from the Ministry of Environmental Protection (MEP). SSDI is responsible for the section Guiyang to Hezhou section (K0+000-K597+650), and FSDI is responsible for the section Hezhou to Guangzhou section (K567+200-K823+513). Accordingly, two separate EIA reports were prepared following relevant provisions specified in Chinese EA laws/regulations and technical guidelines, as well as World Bank safeguard policies. A Consolidated EA and Environmental Management Plan (EMP) 1 in English were prepared by SSDI to synthesize the two separate EIA reports. This Executive Summary is based on these reports, as well as feasibility studies carried out for the project, and karstic cave assessment and cultural resources surveys. -

A Guide to Investment in Hezhou
Positioning A national advanced demonstration zone of circular economy, a new rising industrial city in Guangxi, a regional transportation hub for Guangxi, Guangdong and Hunan, and an outstanding and well-known ecological tourist city in southern China. As “Exploitation of the Works of Nature”, a history book written Name Card in the Ming Dynasty, says "Tin is named He in ancient books, and Linhe (Hezhou) gets its name from the production of tin." Hezhou has a history more than 2,100 years since it was established as Linhe County in 111 B.C., the 6th Yuanding year of Hanwudi in the Western Han National Demonstration Zone of Accepting Industrial Transfer, Chinese National Excellent Tourist City, National Dynasty. In Hejie Town of Babu District, there still stand some Model City of Two-supports, Civilized City of Guangxi, Country of Chinese Hakka People, Longevity Country, Country of well-preserved walls of Nanhan Kingdom built in the period of Five Famous Tea, Country of Rare Stone, and Country of Water Chestnut Dynasties and Ten Kingdoms (907–960 AD). In 226, the 5th Huangwu year of Wu kingdom in the period of Three-kingdoms, Linhe was reestablished as Linhe prefecture. During Environment Overview the period of Sui Dynasty (589–618 AD), Linhe was renamed Hezhou in the 9th Kaihuang year. Located at the place where Guangxi, Guangdong and Hunan provinces meet, Hezhou has been an important business center, since the ancient time, for economic exchanges between Central Plains and the south of the Five Ridges, and for cultural exchange among Hunan, Guangdong, and Guangxi provinces.