Alice C. Levine Editor Physiology, Pathophysiology and Treatment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comp 3, Unit 7 Lecture a Audio Transcript
Audio Transcript Terminology in Healthcare and Public Health Settings Endocrine System Lecture a Health IT Workforce Curriculum Version 4.0/Spring 2016 This material (Comp 3 Unit 7) was developed by the University of Alabama at Birmingham, funded by the Department of Health and Human Services, Office of the National Coordinator for Health Information Technology under Award Number 90WT00007. This work is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/ Slide 1 Welcome to Terminology in Healthcare and Public Health Settings, Endocrine System. This is lecture A, Overview of the Endocrine System, Adrenal Glands and Pancreas. In this lecture, we will be studying the endocrine system. A doctor who treats diseases of the endocrine system is called an endocrinologist. Slide 2 The objectives for the Endocrine System are to: Define, understand and correctly pronounce medical terms related to the endocrine system. Describe common diseases and conditions with an overview of various treatments related to the endocrine system. Slide 3 The endocrine system is composed of eight endocrine glands that assist in regulating our body’s activities. The endocrine glands are found in various locations throughout our body as we will see in a minute. The actions of the endocrine glands also have effects throughout the body. All endocrine glands secrete “hormones,” or chemical messengers, directly into the bloodstream where they are transported to cells that are waiting for their messages. These hormones help your body respond to stress, regulate your blood pressure, and regulate your water and salt balance. -

Diseases of the Digestive System (KOO-K93)
CHAPTER XI Diseases of the digestive system (KOO-K93) Diseases of oral cavity, salivary glands and jaws (KOO-K14) lijell Diseases of pulp and periapical tissues 1m Dentofacial anomalies [including malocclusion] Excludes: hemifacial atrophy or hypertrophy (Q67.4) K07 .0 Major anomalies of jaw size Hyperplasia, hypoplasia: • mandibular • maxillary Macrognathism (mandibular)(maxillary) Micrognathism (mandibular)( maxillary) Excludes: acromegaly (E22.0) Robin's syndrome (087.07) K07 .1 Anomalies of jaw-cranial base relationship Asymmetry of jaw Prognathism (mandibular)( maxillary) Retrognathism (mandibular)(maxillary) K07.2 Anomalies of dental arch relationship Cross bite (anterior)(posterior) Dis to-occlusion Mesio-occlusion Midline deviation of dental arch Openbite (anterior )(posterior) Overbite (excessive): • deep • horizontal • vertical Overjet Posterior lingual occlusion of mandibular teeth 289 ICO-N A K07.3 Anomalies of tooth position Crowding Diastema Displacement of tooth or teeth Rotation Spacing, abnormal Transposition Impacted or embedded teeth with abnormal position of such teeth or adjacent teeth K07.4 Malocclusion, unspecified K07.5 Dentofacial functional abnormalities Abnormal jaw closure Malocclusion due to: • abnormal swallowing • mouth breathing • tongue, lip or finger habits K07.6 Temporomandibular joint disorders Costen's complex or syndrome Derangement of temporomandibular joint Snapping jaw Temporomandibular joint-pain-dysfunction syndrome Excludes: current temporomandibular joint: • dislocation (S03.0) • strain (S03.4) K07.8 Other dentofacial anomalies K07.9 Dentofacial anomaly, unspecified 1m Stomatitis and related lesions K12.0 Recurrent oral aphthae Aphthous stomatitis (major)(minor) Bednar's aphthae Periadenitis mucosa necrotica recurrens Recurrent aphthous ulcer Stomatitis herpetiformis 290 DISEASES OF THE DIGESTIVE SYSTEM Diseases of oesophagus, stomach and duodenum (K20-K31) Ill Oesophagitis Abscess of oesophagus Oesophagitis: • NOS • chemical • peptic Use additional external cause code (Chapter XX), if desired, to identify cause. -

Hormonal and Metabolic Response to Operative Stress in the Neonate
Hormonal and Metabolic Response to Operative Stress in the Neonate DAVID J. SCHMELING, M.D. AND ARNOLD G. CORAN, M.D. From the Section of Pediatric Surgery, Mott Children’s Hospital and University of Michigan Medical School, Ann Arbor, Michigan ABSTRACT. It is evident from this review that newborns, primarily catabolic in nature because the combined hormonal even those born prematurely, are capable of mounting an changes include an increased release of catabolic hormones endocrine and metabolic response to operative stress. Unfor- such as catecholamines, glucagon, and corticosteroids coupled tunately, many of the areas for which a relatively well-charac- with a suppression of and peripheral resistance to the effects terized response exists in adults are poorly documented in of the primary anabolic hormone, insulin. (Journal of Paren- neonates. As is the case in adults, the response seems to be teral and Enteral Nutrition 15:215-238, 1991) It is apparent that adult patients demonstrate a cata- are subjected to greatly increased rates of complications bolic response to the stresses induced by operative or such as cardiac or pulmonary insufficiency, myocardial accidental trauma. It seems that the degree of this cata- infarction, impaired hepatic and/or renal function, gas- bolic response may be quantitatively related to the extent tric stress ulcers, and sepsis. Furthermore, evidence ex- of the trauma or the magnitude of associated complica- ists to suggest that this response may be life threatening tions such as infection. The host response to infection, if the induced catabolic activity remains excessive or traumatic injury, or major operative stress is character- unchecked for a prolonged period. -

Attention-Deficit/Hyperactivity Disorder, Its Pharmacotherapy, And
International Journal of Environmental Research and Public Health Article Attention-Deficit/Hyperactivity Disorder, Its Pharmacotherapy, and Adrenal Gland Dysfunction: A Nationwide Population-Based Study in Taiwan Pin-Han Peng 1, Meng-Yun Tsai 2, Sheng-Yu Lee 3,4 , Po-Cheng Liao 5, Yu-Chiau Shyu 5,6,7,* and Liang-Jen Wang 8,* 1 Department of Psychiatry, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung 833, Taiwan; [email protected] 2 Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung 833, Taiwan; [email protected] 3 Department of Psychiatry, Kaohsiung Veterans General Hospital, Kaohsiung 813, Taiwan; [email protected] 4 Department of Psychiatry, College of Medicine, Graduate Institute of Medicine, School of Medicine, Kaohsiung Medical University, Kaohsiung 807, Taiwan 5 Community Medicine Research Center, Keelung Chang Gung Memorial Hospital, Keelung 204, Taiwan; [email protected] 6 Department of Nursing, Chang Gung University of Science and Technology, Taoyuan City 333, Taiwan 7 Institute of Molecular Biology, Academia Sinica, Taipei 115, Taiwan 8 Department of Child and Adolescent Psychiatry, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung 833, Taiwan * Correspondence: [email protected] (Y.-C.S.); [email protected] (L.-J.W.) Received: 24 March 2020; Accepted: 20 May 2020; Published: 25 May 2020 Abstract: This study aims to examine the co-occurrence rate of attention deficit hyperactivity disorder (ADHD) and adrenal gland disorders, as well as whether pharmacotherapy may affect ADHD patients’ risk of developing adrenal gland disorder. One group of patients newly diagnosed with ADHD (n = 75,247) and one group of age- and gender-matching controls (n = 75,247) were chosen from Taiwan0s National Health Insurance database during the period of January 1999 to December 2011. -

Comparison of Surgical Stress Responses During Spinal and General Anesthesia in Curettage Surgery
Anesth Pain Med. 2014 August; 4(3): e20554. DOI: 10.5812/aapm.20554 Research Article Published online 2014 August 13. Comparison of Surgical Stress Responses During Spinal and General Anesthesia in Curettage Surgery 1,2 1,2 1,2 1,2 Fereshteh Amiri ; Ali Ghomeishi ; Seyed Mohammad Mehdi Aslani ; Sholeh Nesioonpour ; 1,3,* Sara Adarvishi 1Pain Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran 2Department of Anesthesiology, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran 3Student Research Committee, Nursing and Midwifery School, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran *Corresponding author : Sara Adarvishi, Student Research Committee, Nursing and Midwifery School, Imam Khomeini Hospital, Ahvaz Jundishapur University of Medical Sciences, Azadegan Ave., Ahvaz, Iran. Tel: +98-6112220168, Fax: +98-6112220168, E-mail: [email protected] Received: ; Revised: ; Accepted: May 25, 2014 June 14, 2014 July 2, 2014 Background: Response to the surgical stress is an involuntary response to metabolic, autonomic as well as hormonal changes that leads to heart rate and blood pressure fluctuations. Objectives: This study aimed to investigate the effect of general versus spinal anesthesia on blood sugar level and hemodynamic changes in patients undergoing curettage surgery. Patients and Methods: In this randomized clinical trial, 50 patients who were candidate for elective curettage surgery were divided into two groups of general (n = 25) and spinal (n = 25) anesthesia. In both groups, blood glucose level was evaluated 10 minutes before, 20 and 60 minutes after initiation of anesthesia. Also, heart rate and mean arterial blood pressure were evaluated at 10 minutes before, 10, 20, 30, 40, 50 and 60 minutes after intiation of anesthesia. -
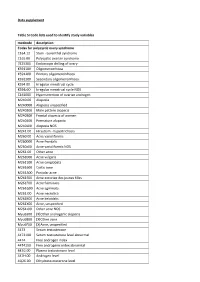
Data Supplement Table SI Code Lists Used to Identify Study Variables
Data supplement Table SI Code lists used to identify study variables medcode description Codes for polycystic ovary syndrome C164.12 Stein - Leventhal syndrome C165.00 Polycystic ovarian syndrome 7E25300 Endoscopic drilling of ovary K591100 Oligomenorrhoea K591200 Primary oligomenorrhoea K591300 Secondary oligomenorrhoea K594.00 Irregular menstrual cycle K594z00 Irregular menstrual cycle NOS C161000 Hypersecretion of ovarian androgen M240.00 Alopecia M240000 Alopecia unspecified M240200 Male pattern alopecia M240300 Frontal alopecia of women M240400 Premature alopecia M240z00 Alopecia NOS M241.00 Hirsutism - hypertrichosis M260.00 Acne varioliformis M260000 Acne frontalis M260z00 Acne varioliformis NOS M261.00 Other acne M261000 Acne vulgaris M261100 Acne conglobata M261600 Cystic acne M261A00 Pustular acne M261E00 Acne excoriee des jeunes filles M261F00 Acne fulminans M261G00 Acne agminata M261J00 Acne necrotica M261K00 Acne keloidalis M261X00 Acne, unspecified M261z00 Other acne NOS Myu6300 [X]Other androgenic alopecia Myu6800 [X]Other acne Myu6F00 [X]Acne, unspecified 4473 Serum testosterone 4473100 Serum testosterone level abnormal 4474 Free androgen index 4474100 Free androgenic index abnormal 447G.00 Plasma testosterone level 447H.00 Androgen level 4Q26.00 Dihydrotestosterone level 4Q2E.00 Free testosterone level 4Q2F.00 Calculated free testosterone ZRBs.00 Ferriman and Galwey score K53..11 Ovarian cysts K532.00 Other ovarian cysts K532z00 Ovarian cyst NOS Kyu9500 [X]Other and unspecified ovarian cysts Codes for diseases for exclusion -

Stress Response to Surgery, Anesthetics Role and Impact On
a & hesi C st lin e ic n a A l f R o e l s Journal of Anesthesia & Clinical e a a n r r c u h o Paola et al., J Anesth Clin Res 2015, 6:7 J ISSN: 2155-6148 Research DOI: 10.4172/2155-6148.1000539 Review Article Open Access Stress Response to Surgery, Anesthetics Role and Impact on Cognition Aceto Paola1*, Lai Carlo2, Dello Russo Cinzia3, Perilli Valter1, Navarra Pierluigi3 and Sollazzi Liliana1 1Department of Anesthesiology and Intensive Care, Agostino Gemelli Hospital, Rome, Italy 2Department of Dynamic and Clinical Psychology, University of Rome “Sapienza”, Rome, Italy 3Department of Pharmacology, Catholic University of Sacred Heart, Rome, Italy *Corresponding author: Paola Aceto, Department of Anaesthesiology and Intensive Care, Catholic University of Sacred Heart, Largo A. Gemelli, 8-00168, Rome, Italy, Tel: +39-06-30154507; Fax: +39-06-3013450; E-mail: [email protected], [email protected] Received date: June 08, 2015, Accepted date: July 08, 2015, Published date: July 13, 2015 Copyright: © 2015 Paola A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract The stress response to surgery includes a number of hormonal changes initiated by neuronal activation of the Hypothalamo-Pituitary-Adrenal (HPA) axis. Surgery is one of the most potent activators of ACTH and cortisol secretion, and increased plasma concentrations of both hormones can be measured few minutes after the start of surgery. -

Stress Dose Steroids: Myths and Perioperative Medicine
Stress Dose Steroids: Myths and Perioperative Medicine Literature Review by Sina Moshiri Introduction Many patients presenting for surgery receive regular doses of glucocorticoids for treatment of systemic autoimmune inflammatory disease, asthma and chronic pulmonary disease, and post organ transplantation Traditionally, supplemental dosing of glucocorticoids prior to surgery was thought to be necessary in these patients to avoid hypotension and shock Alternative strategies were rarely considered, and patients still often receive preoperative steroids despite the known infectious, metabolic and would healing risks associated with glucocorticoids Recommendations for perioperative glucocorticoid management have remained unchallenged despite the relative frequency with which many patients use glucocorticoids, and the little evidence supporting this practice Rationale behind this practice was based on the positive feedback inhibition governing the hypothalamic-pituitary-adrenal (HPA) axis. It was thought that long-term, iatrogenic glucocorticoid administration would result in suppression of the HPA axis and increase risk of adrenal crisis in response to surgical stress. This paper seeks to review the perioperative mechanism and use of glucocorticoids, and review the literature and recommendations on which these practices are based. Adrenal Physiology The body consists of two adrenal glands situated on top of the kidneys. These glands help maintain homeostasis through production of two hormones, cortisol (glucocorticoids) and aldosterone (mineralocorticoid). Adrenal glands are involved in electrolyte and fluid balance, which is regulated by a feedback mechanism. Release of corticotropin releasing hormone (CRH) from hypothalamus, stimulates the release of adrenocorticotropic (ACTH) from the pituitary gland. ACTH triggers the production of cortisol by the adrenal glands. Cortisol is released in response to stress and low blood sugar, and has various metabolic effects on the body. -

Cortisol and Antidiuretic Hormone Responses to Stress in Cardiac Surgical Patients
CORTISOL AND ANTIDIURETIC HORMONE RESPONSES TO STRESS IN CARDIAC SURGICAL PATIENTS YASU OKA, SHIGEHARU WAKAYAMA, TSUTOMU OYAMA, Lou[s R. ORKIN, RONALD M. BECKER, M. DONALD BLAUFOX AND ROBERT W.M. FRATER ABSTRACT The hormonal responses to anaesthesia and cardiac surgery were studied in patients undergoing valve or coronary bypass surgery. Marked increases in antidiuretic hormone levels as a result of surgical stress were seen, and were of approximately equal magnitude in both groups. Although both groups also showed marked increases in plasma cortisol levels in response to operations, this response appeared to be relatively bhmted in valve surgery patients, especially at the end of operation and in the intensive care unit. This blunted cortisol response may be a manifestation of exhaustion of adrenocortical reserves in valvular surgical patients whose sympathoadrenal system has already been chronically stimulatcd by a low output state. The important role of the neuroendocrine system in maintaining homeostasis postopera- tively has long been recognized; this relative cortisol deficiency may be aetiologically related to poor postoperative recovery in critically ill valvular surgery patients. KEY WORDS: CARDIAC SURGERY, stress response. A NUMBER OF INVESTIGATORSI-6 have indicated The present study was undertaken to evaluate that the function of the cardiovascular autonomic whether cortisol and antidiuretic hormone re- nervous system is altered in patients with val- sponses to stress are different in patients with vular heart disease. The loss of sympathetic coronary artery disease and valvular disease, as nervous system control associated with depletion we have previously found in regard to sympathe- of cardiac norepinephrine, when coupled with tic response. -

Stress Hormone Levels in Awake Craniotomy and Craniotomy Under
ogy & N ol eu ur e ro N p h f y o s l Shinoura, et al., J Neurol Neurophysiol 2014, 5:6 i o a l n o r g u y o DOI: 10.4172/2155-9562.1000256 J Journal of Neurology & Neurophysiology ISSN: 2155-9562 Research Article Open Access Stress Hormone Levels in Awake Craniotomy and Craniotomy under General Anesthesia Nobusada Shinoura1*, Ryoji Yamada1, Kaoru Hatori2, Hiroshi Sato2, and Kohei Kimura2 1Department of Neurosurgery, Komagome Metropolitan Hospital, Hon-komagome, Bunkyo-ku, Tokyo, Japan 2Department of Anesthesiology, Komagome Metropolitan Hospital, Hon-komagome, Bunkyo-ku, Tokyo, Japan *Corresponding author: Nobusada Shinoura, Department of Neurosurgery, Komagome Metropolitan Hospital 3-18-22 Hon-komagome, Bunkyo-ku, Tokyo 113-8677, Japan, Tel: +81-3-3823-2101, Fax: +81-3-3824-1552; E-mail: [email protected] Received date: Nov 19, 2014, Accepted date: Dec 16, 2014, Published date: Dec 20, 2014 Copyright: © 2014 Shinoura N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract To compare stress levels between awake craniotomy and craniotomy under general anesthesia, we analyzed plasma levels of adrenaline, cortisol, adrenocorticotropic hormone (ACTH), noradrenaline and dopamine in a large series of patients. Patients who underwent awake craniotomy in our hospital (n=110) were evaluated at 5 sample times: immediately after arterial line insertion (T1); immediately after head fixation in a head frame (T2); 1 h after start of incision (T3); immediately after relief of head fixation (T4); and immediately after arrival in the intensive care unit (T5). -

B Disorders of Autonomic Nervous System
Application of the International Classification of Diseases to Neurology \ Second Edition World Health Organization Geneva 1997 First edition 1987 Second edition 1997 Application of the international classification of diseases to neurology: ICD-NA- 2nd ed. 1. Neurology - classification 2. Nervous system diseases - classification I. Title: ICD-NA ISBN 92 4 154502 X (NLM Classification: WL 15) The World Health Organization welcomes requests for permission to reproduce or translate its publications, in part or in full. Applications and enquiries should be addressed to the Office of Publications, World Health Organization, Geneva, Switzerland, which will be glad to provide the latest information on any changes made to the text, plans for new editions, and reprints and translations already available. ©World Health Organization 1997 Publications of the World Health Organization enjoy copyright protection in accordance with the provisions of Protocol 2 of the Universal Copyright Convention. All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or of certain manufacturers' products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of -

Stress Or Metabolic Response to Surgery and Anesthesia
Review Article http://doi.org/10.18231/j.ijca.2019.031 Stress or metabolic response to surgery and anesthesia Bhavna Gupta1*, Anish Gupta2, Lalit Gupta3 1-3Assistant Professor, 1,3Dept. of Anaesthesia and Critical Care, 2Dept. of CTVS, 1,2All India Institute of Medical Sciences, Rishikesh, Uttarakhand, 3MAMC and Lok Nayak, Hospital, New Delhi, India *Corresponding Author: Bhavna Gupta Email: [email protected] Received: 18th December, 2018 Accepted: 6th March, 2019 Abstract The stress response to surgery, trauma, burns, and critical illness is a well-known entity and encompasses derangements in metabolic and physiological pathways which leads to inflammatory, acute phase, hormonal and genomic responses. There is a state of hyper catabolism and hyper metabolism, which results in impaired wound healing, impaired immune functions and muscle wasting. The stress response to surgery is similar to that induced as a result of traumatic injuries, however it depends on the duration and severity of surgical or traumatic injury. Body responds to such stimuli which may range from minor to massive insults and response is characterized by local or generalized responses. The generalized responses vary from endocrinal, metabolic and biochemical changes in the body and magnitude of the same vary dep/ending on the intensity, severity and duration of stimuli. Stress responses are known to be well tolerated in normal healthy adults, however in patients with known ailments and co-morbidities such as coronary heart disease, hypertension, diabetes, liver diseases, renal insufficiency, old age, changes may be detrimental and life threatening. Keywords: Stress, Metabolic Response, Surgery, Anesthesia. Introduction Hematological and Immunological Response Surgical Stress Response There is a state of fibrinolysis and hypercoagulability Surgery and trauma induce a complex hematological, because of effects of acute phase proteins and cytokines on hormonal, metabolic and immunological responses in the coagulation pathway.