Developmental Biology (Embryology)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

3 Embryology and Development
BIOL 6505 − INTRODUCTION TO FETAL MEDICINE 3. EMBRYOLOGY AND DEVELOPMENT Arlet G. Kurkchubasche, M.D. INTRODUCTION Embryology – the field of study that pertains to the developing organism/human Basic embryology –usually taught in the chronologic sequence of events. These events are the basis for understanding the congenital anomalies that we encounter in the fetus, and help explain the relationships to other organ system concerns. Below is a synopsis of some of the critical steps in embryogenesis from the anatomic rather than molecular basis. These concepts will be more intuitive and evident in conjunction with diagrams and animated sequences. This text is a synopsis of material provided in Langman’s Medical Embryology, 9th ed. First week – ovulation to fertilization to implantation Fertilization restores 1) the diploid number of chromosomes, 2) determines the chromosomal sex and 3) initiates cleavage. Cleavage of the fertilized ovum results in mitotic divisions generating blastomeres that form a 16-cell morula. The dense morula develops a central cavity and now forms the blastocyst, which restructures into 2 components. The inner cell mass forms the embryoblast and outer cell mass the trophoblast. Consequences for fetal management: Variances in cleavage, i.e. splitting of the zygote at various stages/locations - leads to monozygotic twinning with various relationships of the fetal membranes. Cleavage at later weeks will lead to conjoined twinning. Second week: the week of twos – marked by bilaminar germ disc formation. Commences with blastocyst partially embedded in endometrial stroma Trophoblast forms – 1) cytotrophoblast – mitotic cells that coalesce to form 2) syncytiotrophoblast – erodes into maternal tissues, forms lacunae which are critical to development of the uteroplacental circulation. -
Determination of Cell Development, Differentiation and Growth
Pediat. Res. I: 395-408 (1967) Determination of Cell Development, Differentiation and Growth A Review D.M.BROWN[ISO] Department of Pediatrics, University of Minnesota Medical School, Minneapolis, Minnesota, USA Introduction plasmic synthesis, water uptake, or, in the case of intact tissue, intercellular deposition. It may include prolifera It has become increasingly apparent that the basis for tion or the multiplication of identical cells and may be biologic variation in relation to disease states is in accompanied by differentiation which implies anatomical large part predetermined from early embryonic stages. as well as functional changes. The number of cellular Consideration of variations of growth and development units in a tissue may be related to the deoxyribonucleic must take into account the genetic constitution and acid (DNA) content or nucleocytoplasmic units [24, early embryonic events of tissue and organ develop 59, 143]. Differentiation may refer to physical and ment. chemical organization of subcellular components or The incidence of congenital malformation has been to changes in the structure and organization of cells estimated to be 2 to 3 percent of all live born infants and leading to specialized organs. may double by one year [133]. Minor abnormalities are even more common [79]. Furthermore, the large variety of well-defined 'inborn errors of metabolism', Control of Embryonic Chemical Development as well as the less apparent molecular and chromosomal abnormalities, should no doubt be considered as mal Protein syntheses during oogenesis and embryogenesis formations despite the possible lack of gross somatic are guided by nuclear and nucleolar ribonucleic acid aberrations. Low birth weight is a frequent accom (RNA) which are in turn controlled by primer DNA. -

Understanding Paraxial Mesoderm Development and Sclerotome Specification for Skeletal Repair Shoichiro Tani 1,2, Ung-Il Chung2,3, Shinsuke Ohba4 and Hironori Hojo2,3
Tani et al. Experimental & Molecular Medicine (2020) 52:1166–1177 https://doi.org/10.1038/s12276-020-0482-1 Experimental & Molecular Medicine REVIEW ARTICLE Open Access Understanding paraxial mesoderm development and sclerotome specification for skeletal repair Shoichiro Tani 1,2, Ung-il Chung2,3, Shinsuke Ohba4 and Hironori Hojo2,3 Abstract Pluripotent stem cells (PSCs) are attractive regenerative therapy tools for skeletal tissues. However, a deep understanding of skeletal development is required in order to model this development with PSCs, and for the application of PSCs in clinical settings. Skeletal tissues originate from three types of cell populations: the paraxial mesoderm, lateral plate mesoderm, and neural crest. The paraxial mesoderm gives rise to the sclerotome mainly through somitogenesis. In this process, key developmental processes, including initiation of the segmentation clock, formation of the determination front, and the mesenchymal–epithelial transition, are sequentially coordinated. The sclerotome further forms vertebral columns and contributes to various other tissues, such as tendons, vessels (including the dorsal aorta), and even meninges. To understand the molecular mechanisms underlying these developmental processes, extensive studies have been conducted. These studies have demonstrated that a gradient of activities involving multiple signaling pathways specify the embryonic axis and induce cell-type-specific master transcription factors in a spatiotemporal manner. Moreover, applying the knowledge of mesoderm development, researchers have attempted to recapitulate the in vivo development processes in in vitro settings, using mouse and human PSCs. In this review, we summarize the state-of-the-art understanding of mesoderm development and in vitro modeling of mesoderm development using PSCs. We also discuss future perspectives on the use of PSCs to generate skeletal tissues for basic research and clinical applications. -

Embryology J
Embryology J. Matthew Velkey, Ph.D. [email protected] 452A Davison, Duke South Textbook: Langmans’s Medical Embryology, 11th ed. When possible, lectures will be recorded and there may be notes for some lectures, but still NOT a substitute for reading the text. Completing assigned reading prior to class is essential for sessions where a READINESS ASSESSMENT is scheduled. Overall goal: understand the fundamental processes by which the adult form is produced and the clinical consequences that arise from abnormal development. Follicle Maturation and Ovulation Oocytes ~2 million at birth ~40,000 at puberty ~400 ovulated over lifetime Leutinizing Hormone surge (from pituitary gland) causes changes in tissues and within follicle: • Swelling within follicle due to increased hyaluronan • Matrix metalloproteinases degrade surrounding tissue causing rupture of follicle Egg and surrounding cells (corona radiata) ejected into peritoneum Corona radiata provides bulk to facilitate capture of egg. The egg (and corona radiata) at ovulation Corona radiata Zona pellucida (ZP-1, -2, and -3) Cortical granules Transport through the oviduct At around the midpoint of the menstrual cycle (~day 14), a single egg is ovulated and swept into the oviduct. Fertilization usually occurs in the ampulla of the oviduct within 24 hrs. of ovulation. Series of cleavage and differentiation events results in the formation of a blastocyst by the 4th embryonic day. Inner cell mass generates embryonic tissues Outer trophectoderm generates placental tissues Implantation into -

The Derivatives of Three-Layered Embryo (Germ Layers)
HUMANHUMAN EMBRYOLOGYEMBRYOLOGY Department of Histology and Embryology Jilin University ChapterChapter 22 GeneralGeneral EmbryologyEmbryology FourthFourth week:week: TheThe derivativesderivatives ofof trilaminartrilaminar germgerm discdisc Dorsal side of the germ disc. At the beginning of the third week of development, the ectodermal germ layer has the shape of a disc that is broader in the cephalic than the caudal region. Cross section shows formation of trilaminar germ disc Primitive pit Drawing of a sagittal section through a 17-day embryo. The most cranial portion of the definitive notochord has formed. ectoderm Schematic view showing the definitive notochord. horizon =ectoderm hillside fields =neural plate mountain peaks =neural folds Cave sinks into mountain =neural tube valley =neural groove 7.1 Derivatives of the Ectodermal Germ Layer 1) Formation of neural tube Notochord induces the overlying ectoderm to thicken and form the neural plate. Cross section Animation of formation of neural plate When notochord is forming, primitive streak is shorten. At meanwhile, neural plate is induced to form cephalic to caudal end, following formation of notochord. By the end of 3rd week, neural folds and neural groove are formed. Neural folds fuse in the midline, beginning in cervical region and Cross section proceeding cranially and caudally. Neural tube is formed & invade into the embryo body. A. Dorsal view of a human embryo at approximately day 22. B. Dorsal view of a human embryo at approximately day 23. The nervous system is in connection with the amniotic cavity through the cranial and caudal neuropores. Cranial/anterior neuropore Neural fold heart Neural groove endoderm caudal/posterior neuropore A. -

Nephric Lineage Specification by Pax2 and Pax8
Downloaded from genesdev.cshlp.org on September 28, 2021 - Published by Cold Spring Harbor Laboratory Press Nephric lineage specification by Pax2 and Pax8 Maxime Bouchard, Abdallah Souabni, Markus Mandler, Annette Neubüser, and Meinrad Busslinger1 Research Institute of Molecular Pathology, Vienna Biocenter, A-1030 Vienna, Austria The mammalian kidney develops in three successive steps from the initial pronephros via the mesonephros to the adult metanephros. Although the nephric lineage is specified during pronephros induction, no single regulator, including the transcription factor Pax2 or Pax8, has yet been identified to control this initial phase of kidney development. In this paper, we demonstrate that mouse embryos lacking both Pax2 and Pax8 are unable to form the pronephros or any later nephric structures. In these double-mutant embryos, the intermediate mesoderm does not undergo the mesenchymal-epithelial transitions required for nephric duct formation, fails to initiate the kidney-specific expression of Lim1and c -Ret, and is lost by apoptosis 1d after failed pronephric induction. Conversely, retroviral misexpression of Pax2 was sufficient to induce ectopic nephric structures in the intermediate mesoderm and genital ridge of chick embryos. Together, these data identify Pax2 and Pax8 as critical regulators that specify the nephric lineage. [Keywords: Pax2;Pax8;genetic redundancy;kidney development;mesenchymal-epithelial transition;lineage specification] Received June 28, 2002;revised version accepted September 20, 2002. Kidney development in mammals and birds proceeds in development (for review, see Kuure et al. 2000;Davies three successive steps that are all characterized by the and Brändli 2002). The majority of these genes are essen- mesenchymal-to-epithelial transformation of intermedi- tial for proper morphogenesis of the metanephros with ate mesoderm cells. -
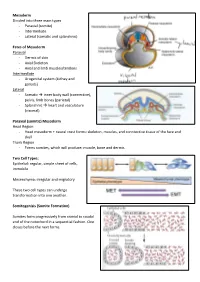
Mesoderm Divided Into Three Main Types - Paraxial (Somite) - Intermediate - Lateral (Somatic and Splanchnic)
Mesoderm Divided into three main types - Paraxial (somite) - Intermediate - Lateral (somatic and splanchnic) Fates of Mesoderm Paraxial - Dermis of skin - Axial Skeleton - Axial and limb muscles/tendons Intermediate - Urogenital system (kidney and gonads) Lateral - Somatic inner body wall (connective), pelvis, limb bones (parietal) - Splanchnic heart and vasculature (visceral) Paraxial (somitic) Mesoderm Head Region - Head mesoderm + neural crest forms: skeleton, muscles, and conntective tissue of the face and skull Trunk Region - Forms somites, which will produce: muscle, bone and dermis Two Cell Types: Epithelial: regular, simple sheet of cells, immobile Mesenchyma: irregular and migratory These two cell types can undergo transformation into one another. Somitogenisis (Somite Formation) Somites form progressively from cranial to caudal end of the notochord in a sequential fashion. One closes before the next forms. Somite Differentiation The somite splits into the epithelial dermamyotome (dermis/muscle) and the messenchymal sclerotome (skeletal). The somite is all paraxial mesoderm. Somite location determines the fate of its associates derma/myo/sclerotomes. Intermediate Mesoderm Urogenital system: - Kidneys - Gonads - Reproductive Duct Systems Runs alongside the paraxial mesoderm. Urogenital System Along mesonephric duct: - Pronephros, mesonephros, and metanephros - Pronephros fall away as gonad develops on ventral-medial side of mesonephros. - Metanephrogenic mesenchyme gives rise to kidney. The mesonephric duct will become the Wolffian duct forming at the nephric bud. The Mullerian duct forms via an invagination on the dorsal side of the nephric duct. The gonad will degenerate one of the two ducts depending on the hormones it produces. XX degenerates Wolffian duct – no testosterone, anti-Mullerian hormone (AMH) not produced, and Mullerian duct can develop in addition to female reproductive organs (ovaries, vagina) XY degenerates Mullerian duct – testosterone, AMH produced, Wolffian duct continues as male reproductive organs (testes, penis) develop. -

Evaluation of Small Molecules for Neuroectoderm Differentiation & Patterning Using Factorial Experimental Design
Evaluation of Small Molecules for Neuroectoderm differentiation & patterning using Factorial Experimental Design Master Thesis in Applied Physics For the degree of Master of Science in Biotechnology DIMITRIOS VOULGARIS Department of Physics, Division of Biological Physics CHALMERS UNIVERSITY OF TECHNOLOGY Göteborg, Sweden 2016 Master thesis in Applied Physics Evaluation of Small Molecules for Neuroectoderm differentiation and patterning using Factorial Experimental Design Dimitrios Voulgaris Department of Physics Division of Biological Physics CHALMERS UNIVERSITY OF TECHNOLOGY Göteborg, Sweden 2016 Evaluation of Small Molecules for Neuroectoderm differentiation and patterning using Factorial Experimental Design DIMITRIOS VOULGARIS © DIMITRIOS VOULGARIS, 2016 Supervisor: Anders Lundin, Industrial PhD candidate, Astra Zeneca and Karolinska Institutet Examiner: Julie Gold, Associate Professor, Division of Biological Physics, Department of Physics, Chalmers University of Technology Master thesis for the degree of M.Sc. in Biotechnology Division of Biological Physics Department of Physics Chalmers University of Technology SE-142 96 Göteborg Sweden Telephone +46 (0)31-722 1000 Cover: hiPSCs differentiated for 4 days on LN-521 in neural induction N2B27 medium stained with DAPI (blue) and the intermediate filament Nestin (green). Printed by Chalmers Reproservice Göteborg, Sweden 2016 Evaluation of Small Molecules for Neuroectoderm differentiation and patterning using Factorial Experimental Design DIMITRIOS VOULGARIS Department of Physics Chalmers University of Technology Evaluation of Small Molecules for Neuroectoderm differentiation and patterning using Factorial Experimental Design DIMITRIOS VOULGARIS Department of Physics Chalmers University of Technology ABSTRACT Screening for therapeutic compounds and treatments for diseases of the Brain does not only encompass the successful generation of iPS-derived homogenous neural stem cell populations but also the capacity of the differentiation protocol to derive on-demand region-specific cells. -

(Serous) Cavities
2003 Veterinary Developmental Anatomy Veterinary Embryology Class Notes (CVM 6100) by Thomas F. Fletcher, DVM, PhD and Alvin F. Weber, DVM, PhD 1 CONTENTS Early Embryogenesis .....................................................3 Musculo-Skeletal Development...................................15 Serous Body Cavities....................................................21 Cardiovascular System ................................................23 Digestive System ...........................................................30 Respiratory System ......................................................36 Urinary System.............................................................39 Genital System ..............................................................42 Face, Nasal Cavity, Mouth, & Pharynx......................47 Nervous System & Special Senses...............................54 Appendix I. Gametogenesis .........................................66 Appendix II. Mitosis and Meiosis ...............................68 Appendix III. List of Anomalies..................................72 2 Early Embryogenesis Embryonic development Embryogenesis, the formation of body structures & organs (organogenesis), requires cell division (proliferation) and cell differentiation (specialization) to produce a variety of cell types and extracellular products. Regulation of gene expression (protein production) is the ultimate explanation for the process of cell differentiation and embryogenesis. Genetic expression will depend on previous genetic history (commitment) -

Embryology and Teratology in the Curricula of Healthcare Courses
ANATOMICAL EDUCATION Eur. J. Anat. 21 (1): 77-91 (2017) Embryology and Teratology in the Curricula of Healthcare Courses Bernard J. Moxham 1, Hana Brichova 2, Elpida Emmanouil-Nikoloussi 3, Andy R.M. Chirculescu 4 1Cardiff School of Biosciences, Cardiff University, Museum Avenue, Cardiff CF10 3AX, Wales, United Kingdom and Department of Anatomy, St. George’s University, St George, Grenada, 2First Faculty of Medicine, Institute of Histology and Embryology, Charles University Prague, Albertov 4, 128 01 Prague 2, Czech Republic and Second Medical Facul- ty, Institute of Histology and Embryology, Charles University Prague, V Úvalu 84, 150 00 Prague 5 , Czech Republic, 3The School of Medicine, European University Cyprus, 6 Diogenous str, 2404 Engomi, P.O.Box 22006, 1516 Nicosia, Cyprus , 4Department of Morphological Sciences, Division of Anatomy, Faculty of Medicine, C. Davila University, Bucharest, Romania SUMMARY Key words: Anatomy – Embryology – Education – Syllabus – Medical – Dental – Healthcare Significant changes are occurring worldwide in courses for healthcare studies, including medicine INTRODUCTION and dentistry. Critical evaluation of the place, tim- ing, and content of components that can be collec- Embryology is a sub-discipline of developmental tively grouped as the anatomical sciences has biology that relates to life before birth. Teratology however yet to be adequately undertaken. Surveys (τέρατος (teratos) meaning ‘monster’ or ‘marvel’) of teaching hours for embryology in US and UK relates to abnormal development and congenital medical courses clearly demonstrate that a dra- abnormalities (i.e. morphofunctional impairments). matic decline in the importance of the subject is in Embryological studies are concerned essentially progress, in terms of both a decrease in the num- with the laws and mechanisms associated with ber of hours allocated within the medical course normal development (ontogenesis) from the stage and in relation to changes in pedagogic methodol- of the ovum until parturition and the end of intra- ogies. -

Early Embryonic Development Till Gastrulation (Humans)
Gargi College Subject: Comparative Anatomy and Developmental Biology Class: Life Sciences 2 SEM Teacher: Dr Swati Bajaj Date: 17/3/2020 Time: 2:00 pm to 3:00 pm EARLY EMBRYONIC DEVELOPMENT TILL GASTRULATION (HUMANS) CLEAVAGE: Cleavage in mammalian eggs are among the slowest in the animal kingdom, taking place some 12-24 hours apart. The first cleavage occurs along the journey of the embryo from oviduct toward the uterus. Several features distinguish mammalian cleavage: 1. Rotational cleavage: the first cleavage is normal meridional division; however, in the second cleavage, one of the two blastomeres divides meridionally and the other divides equatorially. 2. Mammalian blastomeres do not all divide at the same time. Thus the embryo frequently contains odd numbers of cells. 3. The mammalian genome is activated during early cleavage and zygotically transcribed proteins are necessary for cleavage and development. (In humans, the zygotic genes are activated around 8 cell stage) 4. Compaction: Until the eight-cell stage, they form a loosely arranged clump. Following the third cleavage, cell adhesion proteins such as E-cadherin become expressed, and the blastomeres huddle together and form a compact ball of cells. Blatocyst: The descendents of the large group of external cells of Morula become trophoblast (trophoblast produce no embryonic structure but rather form tissues of chorion, extraembryonic membrane and portion of placenta) whereas the small group internal cells give rise to Inner Cell mass (ICM), (which will give rise to embryo proper). During the process of cavitation, the trophoblast cells secrete fluid into the Morula to create blastocoel. As the blastocoel expands, the inner cell mass become positioned on one side of the ring of trophoblast cells, resulting in the distinctive mammalian blastocyst. -

Formation of Germ Layers (Second & Third Week of Development)
8.12.2014 Formation of Germ Layers (Second & Third week of Development) Dr. Archana Rani Associate Professor Department of Anatomy KGMU UP, Lucknow Day 8 • Blastocyst is partially embedded in the endometrial stroma. • Trophoblast differentiates into 2 layers: (i) Cytotrophoblast (ii) Syncytiotrophoblast • Cytotrophoblast shows mitotic division. Day 8 • Cells of inner cell mass (embryoblast) also differentiate into 2 layers: (i) Hypoblast layer (ii) Epiblast layer • Formation of amniotic cavity and embryonic disc. Day 9 • The blastocyst is more deeply embedded in the endometrium. • The penetration defect in the surface epithelium is closed by a fibrin coagulum. Day 9 • Large no. of vacuoles appear in syncytiotrophoblast which fuse to form lacunae which contains embryotroph. Day 9 • Hypoblast forms the roof of the exocoelomic cavity (primary yolk sac). • Heuser’s (exocoelomic membrane) • Extraembryonic mesoderm Day 11 & 12 • Formation of lacunar networks • Extraembryonic coelom (chorionic cavity) • Extraembryonic somatic mesoderm • Extraembryonic splanchnic mesoderm • Chorion Day 13 • Implantation bleeding • Villous structure of trophoblast. • Formation of Primary villi • Secondary (definitive) yolk sac • Chorionic plate (extraembronic mesoderm with cytotrophoblast) Third week of Development • Gastrulation (formation of all 3 germ layers) • Formation of primitive streak • Formation of notochord • Differentiation of 3 germ layers from Bilaminar to Trilaminar germ disc Formation of Primitive Streak (PS) • First sign of gastrulation • On 15th day • Primitive node • Primitive pit • Formation of mesenchyme on 16th day • Formation of embryonic endoderm • Intraembryonic mesoderm • Ectoderm • Epiblast is the source of all 3 germ layers Fate of Primitive Streak • Continues to form mesodermal cells upto early part of 4th week • Normally, the PS degenerates & diminishes in size.