Decapod Crustacean Phylogenetics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Classification of Living and Fossil Genera of Decapod Crustaceans
RAFFLES BULLETIN OF ZOOLOGY 2009 Supplement No. 21: 1–109 Date of Publication: 15 Sep.2009 © National University of Singapore A CLASSIFICATION OF LIVING AND FOSSIL GENERA OF DECAPOD CRUSTACEANS Sammy De Grave1, N. Dean Pentcheff 2, Shane T. Ahyong3, Tin-Yam Chan4, Keith A. Crandall5, Peter C. Dworschak6, Darryl L. Felder7, Rodney M. Feldmann8, Charles H. J. M. Fransen9, Laura Y. D. Goulding1, Rafael Lemaitre10, Martyn E. Y. Low11, Joel W. Martin2, Peter K. L. Ng11, Carrie E. Schweitzer12, S. H. Tan11, Dale Tshudy13, Regina Wetzer2 1Oxford University Museum of Natural History, Parks Road, Oxford, OX1 3PW, United Kingdom [email protected] [email protected] 2Natural History Museum of Los Angeles County, 900 Exposition Blvd., Los Angeles, CA 90007 United States of America [email protected] [email protected] [email protected] 3Marine Biodiversity and Biosecurity, NIWA, Private Bag 14901, Kilbirnie Wellington, New Zealand [email protected] 4Institute of Marine Biology, National Taiwan Ocean University, Keelung 20224, Taiwan, Republic of China [email protected] 5Department of Biology and Monte L. Bean Life Science Museum, Brigham Young University, Provo, UT 84602 United States of America [email protected] 6Dritte Zoologische Abteilung, Naturhistorisches Museum, Wien, Austria [email protected] 7Department of Biology, University of Louisiana, Lafayette, LA 70504 United States of America [email protected] 8Department of Geology, Kent State University, Kent, OH 44242 United States of America [email protected] 9Nationaal Natuurhistorisch Museum, P. O. Box 9517, 2300 RA Leiden, The Netherlands [email protected] 10Invertebrate Zoology, Smithsonian Institution, National Museum of Natural History, 10th and Constitution Avenue, Washington, DC 20560 United States of America [email protected] 11Department of Biological Sciences, National University of Singapore, Science Drive 4, Singapore 117543 [email protected] [email protected] [email protected] 12Department of Geology, Kent State University Stark Campus, 6000 Frank Ave. -

Amphidromy in Shrimps: a Life Cycle Between Rivers and the Sea
Lat. Am. J. Aquat. Res., 41(4): 633-650, 2013 Amphidromy in shrimps: a life cycle 633 “Studies on Freshwater Decapods in Latin America” Ingo S. Wehrtmann & Raymond T. Bauer (Guest Editors) DOI: 103856/vol41-issue4-fulltext-2 Review Amphidromy in shrimps: a life cycle between rivers and the sea Raymond T. Bauer1 1Department of Biology, University of Louisiana at Lafayette, Lafayette, Louisiana, 70504-2451 USA ABSTRACT. Amphidromy is a diadromous life history pattern, common in tropical and subtropical freshwater caridean shrimps, in which adults live, breed and spawn small-sized embryos in freshwater but have extended larval development (ELD) in marine waters. Most completely freshwater species spawn large embryos with either direct or abbreviated larval development (ALD). An important benefit of amphidromy is dispersal among river systems via marine larvae, which increases their access to alternative habitats. Thus, amphidromous species have much broader geographic distributions than closely related completely freshwater ones with ALD. ALD and freshwater ELD species appear to have evolved from amphidromous species with marine ancestors. Delivery of larvae to the sea in many amphidromous species is accomplished by upstream hatching and river drift of larvae to the sea. In other species, the females themselves apparently migrate down to marine waters to spawn. After development, the postlarvae must find a river mouth and migrate upstream to the adult habitat. Migrations occur at night, with juveniles swimming or crawling along the river or stream bank. Larvae are released during the wet or flood season of the year, while juvenile migrations take place during the dry or low-flow season. -

Two Freshwater Shrimp Species of the Genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the Description of a New Species
A peer-reviewed open-access journal ZooKeys 923: 15–32 (2020) Caridina tetrazona 15 doi: 10.3897/zookeys.923.48593 RESEarcH articLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Two freshwater shrimp species of the genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the description of a new species Qing-Hua Chen1, Wen-Jian Chen2, Xiao-Zhuang Zheng2, Zhao-Liang Guo2 1 South China Institute of Environmental Sciences, Ministry of Ecology and Environment, Guangzhou 510520, Guangdong Province, China 2 Department of Animal Science, School of Life Science and Enginee- ring, Foshan University, Foshan 528231, Guangdong Province, China Corresponding author: Zhao-Liang Guo ([email protected]) Academic editor: I.S. Wehrtmann | Received 19 November 2019 | Accepted 7 February 2020 | Published 1 April 2020 http://zoobank.org/138A88CC-DF41-437A-BA1A-CB93E3E36D62 Citation: Chen Q-H, Chen W-J, Zheng X-Z, Guo Z-L (2020) Two freshwater shrimp species of the genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the description of a new species. ZooKeys 923: 15–32. https://doi.org/10.3897/zookeys.923.48593 Abstract A faunistic and ecological survey was conducted to document the diversity of freshwater atyid shrimps of Dawanshan Island. Two species of Caridina that occur on this island were documented and discussed. One of these, Caridina tetrazona sp. nov. is described and illustrated as new to science. It can be easily distinguished from its congeners based on a combination of characters, which includes a short rostrum, the shape of the endopod of the male first pleopod, the segmental ratios of antennular peduncle and third maxilliped, the slender scaphocerite, and the absence of a median projection on the posterior margin. -

BIOPAPUA Expedition Highlighting Deep-Sea Benthic Biodiversity of Papua New- Guinea
Biopapua Expedition – Progress report MUSÉUM NATIONAL D'HISTOIRE NATURELLE 57 rue Cuvier 75005 PARIS‐ France BIOPAPUA Expedition Highlighting deep-sea benthic Biodiversity of Papua New- Guinea Submitted by: Muséum National d'Histoire Naturelle (MNHN) Represented by (co‐PI): Dr Sarah Samadi (Researcher, IRD) Dr Philippe Bouchet (Professor, MNHN) Dr Laure Corbari (Research associate, MNHN) 1 Biopapua Expedition – Progress report Contents Foreword 3 1‐ Our understanding of deep‐sea biodiversity of PNG 4 2 ‐ Tropical Deep‐Sea Benthos program 5 3‐ Biopapua Expedition 7 4‐ Collection management 15 5‐ Preliminary results 17 6‐ Outreach and publications 23 7‐ Appendices 26 Appendix 1 27 NRI, note n°. 302/2010 on 26th march, 2010, acceptance of Biopapua reseach programme Appendix 2 28 Biopapua cruise Report, submitted by Ralph MANA (UPNG) A Report Submitted to School of Natural and Physical Sciences, University of Papua New Guinea Appendix 3 39 Chan, T.Y (2012) A new genus of deep‐sea solenocerid shrimp (Crustacea: Decapoda: Penaeoidea) from the Papua New Guinea. Journal of Crustacean Biology, 32(3), 489‐495. Appendix 4 47 Pante E, Corbari L., Thubaut J., Chan TY, Mana R., Boisselier MC, Bouchet P., Samadi S. (In Press). Exploration of the deep‐sea fauna of Papua New Guinea. Oceanography Appendix 5 60 Richer de Forges B. & Corbari L. (2012) A new species of Oxypleurodon Miers, 1886 (Crustacea Brachyura, Majoidea) from the Bismark Sea, Papua New Guinea. Zootaxa. 3320: 56–60 Appendix 6 66 Taxonomic list: Specimens in MNHN and Taiwan collections 2 Biopapua Expedition – Progress report Foreword Biopapua cruise was a MNHN/IRD deep‐sea cruise in partnership with the School of Natural and Physical Sciences, University of Papua New Guinea. -

Rendimiento Reproductivo De Hembras De Cryphiops Caementarius (Crustacea: Palaemonidae) Mantenidas Con Alimento Natural
Rev. peru. biol. 16(2): 191- 193 (Diciembre 2009) © Facultad de Ciencias Biológicas UNMSM Rendimiento reproductivo de hembrasVersión de Online CRYPHIOPS ISSN C AEMENTARIUS 1727-9933 Rendimiento reproductivo de hembras de Cryphiops caementarius (Crustacea: Palaemonidae) mantenidas con alimento natural Reproductive Performance of female of Cryphiops caementarius (Crustacea: Palaemonidae) maintained with natural food Magali Bazán1, Silvia Gámez1 y Walter Eduardo Reyes2 1 Escuela de Biología en Acui- cultura. Universidad Nacional del Resumen Santa. El objetivo del presente trabajo fue determinar el rendimiento reproductivo de hembras de C. caementarius 2 Departamento de Biología, Micro- biología y Biotecnología. Facultad mantenidas con alimento natural. Se empleó 24 hembras inmaduras (5,2 cm y 2,0 g), acondicionadas en ocho de Ciencias. Universidad Nacional acuarios (45 L) y alimentadas durante dos meses de acuerdo a cada tratamiento, con pota (Dosidicus sp.), del Santa. Av. Universitaria s/n Urb. almeja (Semele solida), poliqueto (Pseudonereis sp.) y con alimento balanceado. El rendimiento reproductivo Bellamar. Nvo. Chimbote. Ancash. Perú. Email Walter Reyes: wre- de las hembras fue mejorado cuando se alimentó con poliqueto y pota, lográndose la maduración entre 16 y [email protected] 18 días con alta fecundidad (2627 y 1377 huevos g-1) y fertilidad (2566 y 1364 larvas g-1, respectivamente). Palabras Claves: Camarón, Cryphiops caementarius, nutrición, reproducción. Abstract The aim was to determine the reproductive performance of females of C. caementarius maintained with natural food. Twenty four females inmature were used (5,2 cm and 2,0 g), conditioned in eight aquarium (50 l) and fed Presentado: 30/10/2009 Aceptado: 26/12/2009 during two months according to each treatment, with giant squid (Dosidicus sp), clam (Semele solid), polychaete Publicado online: 12/01/2010 (Pseudonereis sp.) and with balanced. -
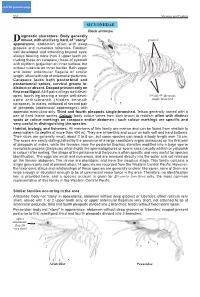
W7192e19.Pdf
click for previous page 952 Shrimps and Prawns Sicyoniidae SICYONIIDAE Rock shrimps iagnostic characters: Body generally Drobust, with shell very hard, of “stony” grooves appearance; abdomen often with deep grooves and numerous tubercles. Rostrum well developed and extending beyond eyes, always bearing more than 3 upper teeth (in- cluding those on carapace); base of eyestalk with styliform projection on inner surface, but without tubercle on inner border. Both upper and lower antennular flagella of similar length, attached to tip of antennular peduncle. 1 Carapace lacks both postorbital and postantennal spines, cervical groove in- distinct or absent. Exopod present only on first maxilliped. All 5 pairs of legs well devel- 2 oped, fourth leg bearing a single well-devel- 3rd and 4th pleopods 4 single-branched oped arthrobranch (hidden beneath 3 carapace). In males, endopod of second pair 5 of pleopods (abdominal appendages) with appendix masculina only. Third and fourth pleopods single-branched. Telson generally armed with a pair of fixed lateral spines. Colour: body colour varies from dark brown to reddish; often with distinct spots or colour markings on carapace and/or abdomen - such colour markings are specific and very useful in distinguishing the species. Habitat, biology, and fisheries: All members of this family are marine and can be found from shallow to deep waters (to depths of more than 400 m). They are all benthic and occur on both soft and hard bottoms. Their sizes are generally small, about 2 to 8 cm, but some species can reach a body length over 15 cm. The sexes are easily distinguished by the presence of a large copulatory organ (petasma) on the first pair of pleopods of males, while the females have the posterior thoracic sternites modified into a large sperm receptacle process (thelycum) which holds the spermatophores or sperm sacs (usually whitish or yellowish in colour) after mating. -

Cryphiops Caementarius (Molina, 1782)
FICHA DE ANTECEDENTES DE ESPECIE Id especie: Nombre Científico: Cryphiops caementarius (Molina, 1782) Nombre Común: Camarón de río del Norte de Chile Reino: Animalia Orden: Decapoda Phyllum/División: Arthropoda Familia: Palaemonidae Clase: Malacostraca Género: Cryphiops Sinonimia: Cancer caementarius , Molina Palaemon Gaudichaudii , Poeppig Cryphiops spinuloso-manus , Dana Bithynis longimana , Philippi Bithynis gaudichaudii , Ortman Bithynis caementarius , Ortman Antecedentes Gen erales: ASPECTOS MORFOLÓGICOS: Animal robusto, de abdomen tan largo y grueso como el cefalotórax, rostrum con cresta dorsal adornada por fila de 6 a 7 dientes gruesos, puede tener dientes a lo largo del borde ventral o carecer completamente de ellos, existiendo ejemplares con todos los estados intermedios relativos a estos extremos (Jara 1994). Primer y segundo par de patas caminadoras con quela o tenaza terminal; el segundo par mucho más grande que el primero y una de las patas de mayor tamaño que la opuesta (Jara 1994). El segundo par de patas del macho es distinto del de la hembra; la mayor anchura de los extremos del segundo segmento abdominal, en proporción a la longitud del abdomen y la relación cefalotoráxica, nos dan la evidencia de un dimorfismo sexual (Castro 1966). El espécimen macho más grande medido en la Colección del Instituto de Zoología (UACh) alcanzó a 59 mm, mientras que el más grande registrado en la literatura alcanzó 67 mm (Jara 1994). Rasgos distintivos ASPECTOS REPRODUCTIVOS: La mayor parte de los ejemplares migran activamente hacia la desembocadura de los ríos para la reproducción , liberar las larvas en los estuarios o zonas del potamon. En cuevas los machos tienen varias hembras que fertilizan después de la muda. -

(Southern Ocean) Hydrothermal Vents: What More Can We Learn from an Ellipse?
Vol. 542: 13–24, 2016 MARINE ECOLOGY PROGRESS SERIES Published January 19 doi: 10.3354/meps11571 Mar Ecol Prog Ser OPENPEN ACCESSCCESS Isotopic niche variability in macroconsumers of the East Scotia Ridge (Southern Ocean) hydrothermal vents: What more can we learn from an ellipse? W. D. K. Reid1,*, C. J. Sweeting2, B. D. Wigham3, R. A. R. McGill4, N. V. C. Polunin5 1Ridley Building, School of Biology, Newcastle University, Newcastle, NE1 7RU, UK 2Marine Management Organisation, Lancaster House, Hampshire Court, Newcastle upon Tyne, NE4 7YH, UK 3Dove Marine Laboratory, School of Marine Science & Technology, Newcastle University, Cullercoats, NE30 4PZ, UK 4NERC Life Sciences Mass Spectrometry Facility, Scottish Universities Environmental Research Centre, East Kilbride, G75 0QF, UK 5Ridley Building, School of Marine Science & Technology, Newcastle University, Newcastle, NE1 7RU, UK ABSTRACT: Aspects of between-individual trophic niche width can be explored through the iso- topic niche concept. In many cases isotopic variability can be influenced by the scale of sampling and biological characteristics including body size or sex. Sample size-corrected (SEAc) and Bayesian (SEAb) standard ellipse areas and generalised least squares (GLS) models were used to explore the spatial variability of δ13C and δ15N in Kiwa tyleri (decapod), Gigantopelta chessoia (peltospirid gastropod) and Vulcanolepas scotiaensis (stalked barnacle) collected from 3 hydrothermal vent field sites (E2, E9N and E9S) on the East Scotia Ridge (ESR), Southern Ocean. SEAb only revealed spatial differences in isotopic niche area in male K. tyleri. However, the parameters used to draw the SEAc, eccentricity (E) and angle of the major SEAc axis to the x-axis (θ), indicated spatial differences in the relationships between δ13C and δ15N in all 3 species. -

Annotated Checklist of New Zealand Decapoda (Arthropoda: Crustacea)
Tuhinga 22: 171–272 Copyright © Museum of New Zealand Te Papa Tongarewa (2011) Annotated checklist of New Zealand Decapoda (Arthropoda: Crustacea) John C. Yaldwyn† and W. Richard Webber* † Research Associate, Museum of New Zealand Te Papa Tongarewa. Deceased October 2005 * Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, New Zealand ([email protected]) (Manuscript completed for publication by second author) ABSTRACT: A checklist of the Recent Decapoda (shrimps, prawns, lobsters, crayfish and crabs) of the New Zealand region is given. It includes 488 named species in 90 families, with 153 (31%) of the species considered endemic. References to New Zealand records and other significant references are given for all species previously recorded from New Zealand. The location of New Zealand material is given for a number of species first recorded in the New Zealand Inventory of Biodiversity but with no further data. Information on geographical distribution, habitat range and, in some cases, depth range and colour are given for each species. KEYWORDS: Decapoda, New Zealand, checklist, annotated checklist, shrimp, prawn, lobster, crab. Contents Introduction Methods Checklist of New Zealand Decapoda Suborder DENDROBRANCHIATA Bate, 1888 ..................................... 178 Superfamily PENAEOIDEA Rafinesque, 1815.............................. 178 Family ARISTEIDAE Wood-Mason & Alcock, 1891..................... 178 Family BENTHESICYMIDAE Wood-Mason & Alcock, 1891 .......... 180 Family PENAEIDAE Rafinesque, 1815 .................................. -

Deep-Sea Life Issue 14, January 2020 Cruise News E/V Nautilus Telepresence Exploration of the U.S
Deep-Sea Life Issue 14, January 2020 Welcome to the 14th edition of Deep-Sea Life (a little later than anticipated… such is life). As always there is bound to be something in here for everyone. Illustrated by stunning photography throughout, learn about the deep-water canyons of Lebanon, remote Pacific Island seamounts, deep coral habitats of the Caribbean Sea, Gulf of Mexico, Southeast USA and the North Atlantic (with good, bad and ugly news), first trials of BioCam 3D imaging technology (very clever stuff), new deep pelagic and benthic discoveries from the Bahamas, high-risk explorations under ice in the Arctic (with a spot of astrobiology thrown in), deep-sea fauna sensitivity assessments happening in the UK and a new photo ID guide for mesopelagic fish. Read about new projects to study unexplored areas of the Mid-Atlantic Ridge and Azores Plateau, plans to develop a water-column exploration programme, and assessment of effects of ice shelf collapse on faunal assemblages in the Antarctic. You may also be interested in ongoing projects to address and respond to governance issues and marine conservation. It’s all here folks! There are also reports from past meetings and workshops related to deep seabed mining, deep-water corals, deep-water sharks and rays and information about upcoming events in 2020. Glance over the many interesting new papers for 2019 you may have missed, the scientist profiles, job and publishing opportunities and the wanted section – please help your colleagues if you can. There are brief updates from the Deep- Ocean Stewardship Initiative and for the deep-sea ecologists amongst you, do browse the Deep-Sea Biology Society president’s letter. -

Evolutionary Transformations of the Reproductive System in Eubrachyura (Crustacea: Decapoda)
EVOLUTIONARY TRANSFORMATIONS OF THE REPRODUCTIVE SYSTEM IN EUBRACHYURA (CRUSTACEA: DECAPODA) DISSERTATION zur Erlangung des akademischen Grades Doctor rerum naturalium (Dr. rer. nat.) eingereicht an der Lebenswissenschaftlichen Fakultät der Humboldt-Universität zu Berlin von M. Sc. Katja, Kienbaum, geb. Jaszkowiak Präsidentin der Humboldt-Universität zu Berlin Prof. Dr.-Ing. Dr. Sabine Kunst Dekan der Lebenswissenschaftlichen Fakultät der Humboldt-Universität zu Berlin Prof. Dr. Bernhard Grimm Gutachter 1. Prof. Dr. Gerhard Scholtz 2. PD Dr. Thomas Stach 3. PD Dr. Christian Wirkner Tag der mündlichen Prüfung: 03.05.2019 CONTENT C ONTENT A BSTRACT v i - vii Z USAMMENFASSUNG viii - x 1 | INTRODUCTION 1 - 11 1.1 | THE BRACHYURA 1 1.1.1 | OBJECT OF INVESTIGATION 1 - 5 1.1.2 | WHAT WE (DO NOT) KNOW ABOUT THE PHYLOGENY OF EUBRACHURA 6 - 10 1. 2 |MS AI 10 - 11 2 | THE MORPHOLOGY OF THE MALE AND FEMALE REPRODUCTIVE SYSTEM IN TWO 12 - 34 SPECIES OF SPIDER CRABS (DECAPODA: BRACHYURA: MAJOIDEA) AND THE ISSUE OF THE VELUM IN MAJOID REPRODUCTION. 2.1 | INTRODUCTION 13 - 14 2.2 | MATERIAL AND METHODS 14 - 16 2.3 | RESULTS 16 - 23 2.4 | DISCUSSION 24 - 34 3 | THE MORPHOLOGY OF THE REPRODUCTIVE SYSTEM IN THE CRAB 35 - 51 PERCNON GIBBESI (DECAPODA: BRACHYURA: GRAPSOIDEA) REVEALS A NEW COMBINATION OF CHARACTERS. 3.1 | INTRODUCTION 36 - 37 3.2 | MATERIAL AND METHODS 37 - 38 3.3 | RESULTS 39 - 46 3.4 | DISCUSSION 46 - 51 4 | THE REPRODUCTIVE SYSTEM OF LIMNOPILOS NAIYANETRI INDICATES A 52 - 64 THORACOTREME AFFILIATION OF HYMENOSOMATIDAE (DECAPODA, EUBRACHYURA). -

An Annotated Checklist of the Marine Macroinvertebrates of Alaska David T
NOAA Professional Paper NMFS 19 An annotated checklist of the marine macroinvertebrates of Alaska David T. Drumm • Katherine P. Maslenikov Robert Van Syoc • James W. Orr • Robert R. Lauth Duane E. Stevenson • Theodore W. Pietsch November 2016 U.S. Department of Commerce NOAA Professional Penny Pritzker Secretary of Commerce National Oceanic Papers NMFS and Atmospheric Administration Kathryn D. Sullivan Scientific Editor* Administrator Richard Langton National Marine National Marine Fisheries Service Fisheries Service Northeast Fisheries Science Center Maine Field Station Eileen Sobeck 17 Godfrey Drive, Suite 1 Assistant Administrator Orono, Maine 04473 for Fisheries Associate Editor Kathryn Dennis National Marine Fisheries Service Office of Science and Technology Economics and Social Analysis Division 1845 Wasp Blvd., Bldg. 178 Honolulu, Hawaii 96818 Managing Editor Shelley Arenas National Marine Fisheries Service Scientific Publications Office 7600 Sand Point Way NE Seattle, Washington 98115 Editorial Committee Ann C. Matarese National Marine Fisheries Service James W. Orr National Marine Fisheries Service The NOAA Professional Paper NMFS (ISSN 1931-4590) series is pub- lished by the Scientific Publications Of- *Bruce Mundy (PIFSC) was Scientific Editor during the fice, National Marine Fisheries Service, scientific editing and preparation of this report. NOAA, 7600 Sand Point Way NE, Seattle, WA 98115. The Secretary of Commerce has The NOAA Professional Paper NMFS series carries peer-reviewed, lengthy original determined that the publication of research reports, taxonomic keys, species synopses, flora and fauna studies, and data- this series is necessary in the transac- intensive reports on investigations in fishery science, engineering, and economics. tion of the public business required by law of this Department.