Studies on Metabolism of 1,4-Dioxane, March 2010
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

162 Part 175—Indirect Food Addi
§ 174.6 21 CFR Ch. I (4–1–19 Edition) (c) The existence in this subchapter B Subpart B—Substances for Use Only as of a regulation prescribing safe condi- Components of Adhesives tions for the use of a substance as an Sec. article or component of articles that 175.105 Adhesives. contact food shall not be construed as 175.125 Pressure-sensitive adhesives. implying that such substance may be safely used as a direct additive in food. Subpart C—Substances for Use as (d) Substances that under conditions Components of Coatings of good manufacturing practice may be 175.210 Acrylate ester copolymer coating. safely used as components of articles 175.230 Hot-melt strippable food coatings. that contact food include the fol- 175.250 Paraffin (synthetic). lowing, subject to any prescribed limi- 175.260 Partial phosphoric acid esters of pol- yester resins. tations: 175.270 Poly(vinyl fluoride) resins. (1) Substances generally recognized 175.300 Resinous and polymeric coatings. as safe in or on food. 175.320 Resinous and polymeric coatings for (2) Substances generally recognized polyolefin films. as safe for their intended use in food 175.350 Vinyl acetate/crotonic acid copoly- mer. packaging. 175.360 Vinylidene chloride copolymer coat- (3) Substances used in accordance ings for nylon film. with a prior sanction or approval. 175.365 Vinylidene chloride copolymer coat- (4) Substances permitted for use by ings for polycarbonate film. 175.380 Xylene-formaldehyde resins con- regulations in this part and parts 175, densed with 4,4′-isopropylidenediphenol- 176, 177, 178 and § 179.45 of this chapter. -

A Brief Guide to Authors
Latvian Journal of Chemistry, No. 1/2, 2011, 139–144. DOI 10.2478/v10161-011-0059-3 CYCLODEHYDRATION OF DIOLS IN ACIDIC IONIC LIQUIDS E. Ausekle, A. Priksane, A. Zicmanis Faculty of Chemistry, University of Latvia, Kr. Valdemara Str. 48, Riga, LV-1013 e-mail: [email protected] In the presence of sulfonic acid group functionalized Bronsted-acidic ionic liquids, cyclodehydration of 1,2-ethanediol and 1,4-butanediol is investigated. The role of structure and catalytic activity of ionic liquids on the formation of cyclic ethers: 1,4-dioxane and tetrahydrofuran – is de- termined. Key words: acidic ionic liquids, ethylene glycol, 1,4-butanediol, cyclodehydration, 1,4-dioxane, tetrahydrofuran. INTRODUCTION During the last decades, ionic liquids are extensively used in organic syn- thesis as solvents and catalysts [1, 2]. Bronsted-acidic ionic liquids (AILs) are used as substitutes of mineral and organic acids in acid-catalyzed reactions like esterification, etherification, pinacol–pinacolone rearrangement, condensation etc. [3–6]. Bronsted-acidic ionic liquids have good proton donor properties comparable with mineral acids. AILs as catalysts are non-corrosive, non-volati- le, immiscible with many organic solvents, and they can be recycled and reused [7]. To determine their productivity in diol cyclodehydration, two diols: 1,2- ethanediol (ethylene glycol) and 1,4-butanediol are used. Cyclodehydration of both diols leads to formation of industrially significant cyclic ethers: 1,4-di- oxane and tetrahydrofuran (THF). 1,4-Dioxane and THF are widely used as organic solvents. Tetrahydrofuran is a useful solvent for organic synthesis, production of natural and synthetic resins, and also is a valuable intermediate in manufacture of a number of chemicals and plastics. -

1,4-Dioxane Priority Existing Chemical No
National Industrial Chemicals Notification and Assessment Scheme 1,4-Dioxane Priority Existing Chemical No. 7 __________________________________ Full Public Report June 1998 © Commonwealth of Australia 1998 ISBN 0 642 47104 5 This work is copyright. Apart from any use permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission from AusInfo. Requests and inquiries concerning reproduction and rights should be addressed to the Manager, Legislative Services, AusInfo, GPO Box 84, Canberra, ACT 2601. ii Priority Existing Chemical Number 7 Preface This assessment was carried out under the National Industrial Chemicals Notification and Assessment Scheme (NICNAS). This Scheme was established by the Industrial Chemicals (Notification and Assessment) Act 1989 (the Act), which came into operation on 17 July 1990. The principal aim of NICNAS is to aid in the protection of people at work, the public and the environment from the harmful effects of industrial chemicals, by assessing the risks associated with these chemicals. NICNAS is administered by the National Occupational Health and Safety Commission (NOHSC) and assessments are carried out in conjunction with Environment Australia (EA) and the Therapeutic Goods Administration (TGA), who carry out the environmental and public health assessments, respectively. NICNAS has two major programs: one focusing on the risks associated with new chemicals prior to importation or manufacture; and the other focussing on existing chemicals already in use in Australia. As there are many thousands of existing industrial chemicals in use in Australia, NICNAS has an established mechanism for prioritising and declaring chemicals as Priority Existing Chemicals (PECs). This Full Public PEC report has been prepared by the Director (Chemicals Notification and Assessment) in accordance with the Act. -

Gasket Chemical Services Guide
Gasket Chemical Services Guide Revision: GSG-100 6490 Rev.(AA) • The information contained herein is general in nature and recommendations are valid only for Victaulic compounds. • Gasket compatibility is dependent upon a number of factors. Suitability for a particular application must be determined by a competent individual familiar with system-specific conditions. • Victaulic offers no warranties, expressed or implied, of a product in any application. Contact your Victaulic sales representative to ensure the best gasket is selected for a particular service. Failure to follow these instructions could cause system failure, resulting in serious personal injury and property damage. Rating Code Key 1 Most Applications 2 Limited Applications 3 Restricted Applications (Nitrile) (EPDM) Grade E (Silicone) GRADE L GRADE T GRADE A GRADE V GRADE O GRADE M (Neoprene) GRADE M2 --- Insufficient Data (White Nitrile) GRADE CHP-2 (Epichlorohydrin) (Fluoroelastomer) (Fluoroelastomer) (Halogenated Butyl) (Hydrogenated Nitrile) Chemical GRADE ST / H Abietic Acid --- --- --- --- --- --- --- --- --- --- Acetaldehyde 2 3 3 3 3 --- --- 2 --- 3 Acetamide 1 1 1 1 2 --- --- 2 --- 3 Acetanilide 1 3 3 3 1 --- --- 2 --- 3 Acetic Acid, 30% 1 2 2 2 1 --- 2 1 2 3 Acetic Acid, 5% 1 2 2 2 1 --- 2 1 1 3 Acetic Acid, Glacial 1 3 3 3 3 --- 3 2 3 3 Acetic Acid, Hot, High Pressure 3 3 3 3 3 --- 3 3 3 3 Acetic Anhydride 2 3 3 3 2 --- 3 3 --- 3 Acetoacetic Acid 1 3 3 3 1 --- --- 2 --- 3 Acetone 1 3 3 3 3 --- 3 3 3 3 Acetone Cyanohydrin 1 3 3 3 1 --- --- 2 --- 3 Acetonitrile 1 3 3 3 1 --- --- --- --- 3 Acetophenetidine 3 2 2 2 3 --- --- --- --- 1 Acetophenone 1 3 3 3 3 --- 3 3 --- 3 Acetotoluidide 3 2 2 2 3 --- --- --- --- 1 Acetyl Acetone 1 3 3 3 3 --- 3 3 --- 3 The data and recommendations presented are based upon the best information available resulting from a combination of Victaulic's field experience, laboratory testing and recommendations supplied by prime producers of basic copolymer materials. -

Diethylene Glycol
WORKPLACE ENVIRONMENTAL EXPOSURE LEVEL (2016) Diethylene Glycol I. IDENTIFICATION(1,5) manufacturing; lacquer industry; for industrial drying of gases; monomer for polyester resins and polyester polyols. Chemical Name: 2,2'-Oxybisethanol Synonyms: DEG; Diethylene Glycol; Ethylene Diglycol; 2,2' IV. ANIMAL TOXICOLOGY DATA Oxydiethanol; 2-(2-Hydroxyethoxy) Ethanol; 2,2'- Dihydroxydiethyl Ether A. Acute Toxicity and Irritancy CAS Number: 111-46-6 1. Lethality Data Molecular Formula: C4H10O3 Structural Formula: Species Route LD50 (g/kg) Mouse Oral 13.30-28.23(6-8) Rat Oral 16.56-30.21(6-11) Guinea Pigs Oral 8.68-14.00(6,8,9) Dog Oral 11.19(6) Rabbit Oral 2.69-4.92(6,8) (1-5) II. CHEMICAL AND PHYSICAL PROPERTIES Rabbit Dermal 12.5-13.3(11,17) Physical State and Appearance: Colorless viscous liquid Odor Description: No data available Oral (gavage) administration of 15 ml/kg DEG (16.76 g/kg) to (12,13) Odor Threshold: No data available 30 male Wistar rats was lethal to 20 animals within 5 days. Molecular Weight: 106.12 2. Eye Irritation Conversion Factors: 1 mg/m3 = approx. 0.227 ppm; 3 Undiluted DEG (volume not specified) instilled into the 1 ppm = approx. 4.403 mg/m conjunctival sac of rabbits, dogs, and cats produced no visible Density: 1.119 g/mL at 20°C (68°F) irritation reactions and had no effect on pupillary reaction or Boiling Point: 245°C (473°F) at 760 mmHg corneal reflexes.(7) Instillation of 0.5 ml DEG into the conjunc- Melting Point: -6.5°C (-20.3°F) tival sac of the rabbit produced little or no irritation.(18) Vapor Pressure: 0.01 mmHg at 20°C (68°F), 1 mmHg at 92°C Instillation of 0.1 ml DEG into the eyes of rabbits produced Saturated Vapor Conc: 13 ppm at 20°C (68°F) minor to moderate conjunctival irritation but no corneal injury Flash Point: 138°C (280°F) (Pensky-Martens closed cup) or iritis. -

Alcohols & Glycols Kleinschmidt
8/13/14 Alcohols," Glycols, &" “Cat”cols Kurt Kleinschmidt, MD Section Chief and Program Director, Medical Toxicology UT Southwestern Medical Center Dallas, Texas Alcohols and Glycols • “Iso” means branching of carbon chain • “Glycol” means 2 hydroxyl groups • Ethylene glycol Antifreeze • Propylene glycol Refrigerant • Polyalkylene glycol Refrigerant oil • Physiochemical behavior • If small hydrocarbon group, acts like water • If large hydrocarbon group, acts like the HC-group Alcohols and Glycols: Glycol Ethers • Clear, Syrupy liquid; Inoffensive odors; Low Vapor pressure; Non-flammable • Water & Organic soluble … Very Nice!...”Couplers”! • Do not bioaccumulate b/c undergo rapid hydrolysis • Rapid Dermal, inhalation, and oral absorption • Molecular Weight êèé Dermal absorption • Uses: Solvents Household cleaning products (windows) Humectant and plasticizer Semiconductor industry Brake fluid Diluent Deicers Paints and Coatings 1 8/13/14 Alcohols and Glycols: Glycol Ethers • Two groups: EG Monoalkyl Ethers base: • Ethylene glycol ethers R1OCH2CH2OR2 • Propylene glycol ethers R1=Alkyl gp; R2=H or Acetate • Ethylene Glycol Ethers • Many exist Ethylene Glycol • 2 examples……………. Methyl Ether (EGME) Ethers: R1-O-R2 Ethylene Glycol • Propylene Glycol Ethers Butyl Ether (EGBE) • Many • Example Is a 2o alcohol Propylene Glycol (On the 2nd Carbon) Monomethyl Ether Alcohols and Glycols: " Glycol Ethers Metabolism • ADH is key one: è Alkoxyacetic acids • Toxic Metabolite è Reproductive Problems Ethylene • Gap Acidosis Glycol • Minor route & Debatableè ethylene glycol Ether • Oxaluria seen after some methoxyethanol & butoxyethanol ingestions • But… Ether linkage is fairly stable Is No direct evidence to support Propylene • Its 2o –OH è ADH does NOT metabolize Glycol • CYP Metabolism è CO2 (Non-Toxic) Ethers • Replacing the ethylene glycol ethers Alcohols and Glycols " Clinical Glycol Ethers • Reproductive Not/Less • Animal studies è Reproduction Injury (Spont. -

Reassessment of 3 Tolerance Exemptions for Ethylene Glycol
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY - +,TE* sr4, WASHINGTON, D.C. 20460 Q c, OFFICE OF PREVENTION, PESTICIDES, AND TOXIC SUBSTANCES DATE: June 29,2006 ACTION MEMORANDUM SUBJECT: Reassessment of 3 Tolerance Exemptions for Ethylene Glycol, Diethylene Glycol, and the Combination of Diethylene Glycol Monomethyl Ether, Diethylene Glycol Monoethyl Ether, and Diethylene Glycol Monobutyl Ether FROM: Pauline Wagner, Chief F b.~!!<Lo 'v \ 3~~10 b Inert Ingredient Assessment Branch Registration Division (7505P) TO: Lois A. Rossi, Director Registration Division (7505P) 1. FQPA REASSESSMENT ACTION Action: Reassessment of three inert exemptions from the requirement of a tolerance. The reassessment decision is to maintain the inert tolerance exemptions "as-is." Table 1. Tolerance Exemptions Being Reassessed in this Document CM~fl,~aa,it Appeara in the CFR CAS iT01muw Registry Number @.I@,- Bxemption $in$@ Uses Name %SOa ,. Exprmsion. Antifreeze, deactivator for all pesticides 107-21-1 920 Ethylene glycol - - - used before crop emerges from soil and in 1,2-Ethanediol herbicides before or after crop emerges Deactivator, adjuvant for formulations used before crop emerges from soil and 11 1-46-6 920 Diethylene glycol --- deactivator for formulations used before Ethanol, 2,2'-oxybis- (9CI) crop emerges from soil, stabilizer Diethylene glycol 1 11-77-3 monomethyl ether Ethanol, 2-(2-methoxyethoxy)- 920 Diethylene glycol monoethyl - - - Deactivator for formulations used before 1 1 1-90-0 ether crop emerges from soil, stabilizer Ethanol, 2-(2-ethoxyethoxy)- Diethylene glycol monobutyl 112-34-5 ether Ethanol, 2-(2-butoxyethoxy)- a. Residues listed in 40 CFR 180.920 are exempted from the requirement of a tolerance when used in accordance with good agricultural practice as inert (or occasionally active) ingredients in pesticide formulations applied to growing crops only. -

Locating and Estimating Sources of Ethylene Oxide
United States Office of Air Quality EPA-450/4-84-007L Environmental Protection Planning And Standards Agency Research Triangle Park, NC 27711 September 1986 AIR EPA LOCATING AND ESTIMATING AIR EMISSIONS FROM SOURCES OF ETHYLENE OXIDE L &E EPA- 450/4-84-007L September 1986 LOCATING AND ESTIMATING AIR EMISSIONS FROM SOURCES OF ETHYLENE OXIDE U.S. Environmental Protection Agency Office of Air and Radiation Office of Air Quality Planning and Standards Research Triangle Park, North Carolina 27711 This report has been reviewed by the Office of Air Quality Planning and Standards, U.S. Environmental Protection Agency, and approved for publication as received from the contractor. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, neither does mention of trade names or commercial products constitute endorsement or recommendation for use. EPA - 450/4-84-007L TABLE OF CONTENTS Section Page 1 Purpose of Document .......................................... 1 2 Overview of Document Contents ................................ 3 3 Background ................................................... 5 Nature of Pollutant .................................... 5 Overview of Production and Use ......................... 7 References for Section 3 .............................. 14 4 Emissions from Ethylene Oxide Production .................... 16 Ethylene Oxide Production ................................... 16 References for Section 4 .................................... 33 5 Emissions from Industries Which Use Ethylene -

Detection of Diethylene Glycol in Glycerin and Propylene Glycol by Using High Performance Thin Layer Chromatography HPTLC M.Ph
IOSR Journal of Pharmacy Vol. 1, Issue 1, Nov.-Dec, 2012, pp. 029-034 Detection of Diethylene Glycol in Glycerin and Propylene Glycol by using high performance thin layer chromatography HPTLC M.Ph. Huda Ghanem, Prof. Mhd Ammar Alkhayat, Prof. Mhd Amer Almardini Department of pharmaceutical chemistry and quality control, College of Pharmacy, Damascus University, Syria Abstract: We were developed analytical method rapid, sensitive and easy to use to detect Diethylene glycol (DEG) in some of excipients: Glycerin, and propylene glycol by using high-performance thin-layer chromatography HPTLC with plate fluorinated: HPTLC Plate silica gel 60 F 254, and with mobile phase: acetone: toluene: 5 M ammonium hydroxide at rates 85: 5:10, respectively. And Use a scanner with wavelength: 325 nm. This method allows the detection and assay of toxic Diethylene glycol in excipients: glycerin and propylene glycol in concentrations of not less than 0.1%. This method was verified the validity of it to conduct all the constitutional requirements. Keywords: Diethylene Glycol (DEG), Propylene Glycol (PG), Glycerin, Ethylene Glycol (EG, High Performance Thin Layer Chromatography (HPTLC). Introduction: (DEG( Diethylene Glycol : DEG: ( HO-CH2-CH2-O-CH2-CH2-OH( Diethylene glycol is Organic solvent has many industrial uses (1,2). DEG is classified as toxic material, it causes when dealing with multiple systemic disorders until the occurrence of acute kidney failure and death (3,4,5). Diethylene glycol has physical and chemical properties close to the properties of glycerin and propylene glycol, which is cheaper than both glycerin and propylene glycol, thus forcing some producers and sellers to cheat them with DEG (6,7,2). -

Diethylene Glycol
Scientific Committee on Consumer Products SCCP OPINION ON Diethylene glycol The SCCP adopted this opinion at its 16th plenary of 24 June 2008 SCCP/1181/08 Opinion on diethylene glycol About the Scientific Committees Three independent non-food Scientific Committees provide the Commission with the scientific advice it needs when preparing policy and proposals relating to consumer safety, public health and the environment. The Committees also draw the Commission's attention to the new or emerging problems which may pose an actual or potential threat. They are: the Scientific Committee on Consumer Products (SCCP), the Scientific Committee on Health and Environmental Risks (SCHER) and the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) and are made up of external experts. In addition, the Commission relies upon the work of the European Food Safety Authority (EFSA), the European Medicines Evaluation Agency (EMEA), the European Centre for Disease prevention and Control (ECDC) and the European Chemicals Agency (ECHA). SCCP Questions concerning the safety of consumer products (non-food products intended for the consumer). In particular, the Committee addresses questions related to the safety and allergenic properties of cosmetic products and ingredients with respect to their impact on consumer health, toys, textiles, clothing, personal care products, domestic products such as detergents and consumer services such as tattooing. Scientific Committee members Claire Chambers, Gisela Degen, Ruta Dubakiene, Bozena Jazwiec-Kanyion, -

Ethylene Glycol
ETHYLENE GLYCOL Introduction [1]: Glycols are dihydric alcohols having an aliphatic carbon chain. They have the general chemical formula CnH2n(OH)2. Ethylene glycol is the simplest and the most important of the glycols. It is a colorless, nearly odorless, sweet-tasting, hygroscopic liquid. The chemical formula of ethylene glycol is: C2H6O2. Ethylene glycol was first produced in 1859 by Wurtz. He saponified ethylene glycol diacetate with potassium hydroxide. Three years later he made ethylene glycol by hydration of ethylene oxide. This glycol encounted a commercial importance in 1925 when it was first manufactured in large scale quantities from ethylene oxide through the intermediate ethylene chlorohydrin. Some of the physical properties of the ethylene glycol are shown in Table 1.1. Ethylene glycol Molecular weight, g/gmol 62.07 Freezing point, 0C -13 Boiling point, 0C 197.2 Solubility at 200C., wt.% In water Complete Water in Complete Table 1.1 Physical properties of Ethylene glycol Ethylene glycol is relatively nonvolatile and viscous. It is also soluble in common alcohols and phenol. Manufacture [1]: I. Most of the ethylene glycol produced today is obtained by hydration of ethylene oxide. The reaction is: CH2-O-CH2 + H2O Þ CH2-OH-CH2-OH Ethylene oxide is readily converted to ethylene glycol by either of the following methods: - by the action of a dilute aqueous solution of a strong acid - by reaction with water at elevated temperature and pressure The ethylene glycol which results from these methods is concentrated by evaporations and further purified by vacuum distillations. II. A second commercial method of producing ethylene glycol is based on the reaction of formaldehyde and a mixture of carbon monoxide and water at high pressure and temperature to produce glycolic acid. -
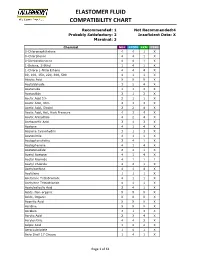
Elastomer Fluid Compatibility Chart
ELASTOMER FLUID COMPATIBILITY CHART Recommended: 1 Not Recommended:4 Probably Satisfactory: 2 Insuficient Data: X Marginal: 3 Chemical NBR EPDM FKM PTFE 0-Chloronaphthalene 4 4 1 X 0-Chlorphenol 4 4 1 X 0-Dichlorobenzene 4 4 1 X 1-Butene, 2-Ethyl 1 4 1 X 1-Chloro 1-Nitro Ethane 4 4 4 X 90, 100, 150, 220, 300, 500 4 1 1 X Abietic Acid X X X X Acetaldehyde 3 2 4 X Acetamide 1 1 3 X Acetanilide 3 1 3 X Acetic Acid 5% 2 1 1 X Acetic Acid, 30% 2 1 3 X Acetic Acid, Glacial 2 2 4 X Acetic Acid, Hot, High Pressure 4 3 4 X Acetic Anhydride 4 2 4 X Acetoacetic Acid 3 1 3 X Acetone 4 1 4 X Acetone Cyanohydrin 3 1 3 X Acetonitrile 3 1 1 X Acetophenetidine 2 4 1 X Acetophenone 4 1 4 X Acetotoluidide 2 4 1 X Acetyl Acetone 4 1 4 X Acetyl Bromide 4 1 1 1 Acetyl Chloride 4 4 1 X Acetylacetone 4 1 4 X Acetylene 1 1 1 X Acetylene Tetrabromide 4 1 1 X Acetylene Tetrachloride 4 1 1 X Acetylsalicylic Acid 2 4 1 X Acids, Non-organic X X X X Acids, Organic X X X X Aconitic Acid X X X X Acridine X X X X Acrolein 3 1 3 X Acrylic Acid 2 3 4 X Acrylonitrile 4 4 3 X Adipic Acid 1 2 2 X Aero Lubriplate 1 4 1 X Aero Shell 17 Grease 1 4 1 X Page 1 of 61 ELASTOMER FLUID COMPATIBILITY CHART Recommended: 1 Not Recommended:4 Probably Satisfactory: 2 Insuficient Data: X Marginal: 3 Chemical NBR EPDM FKM PTFE Aero Shell 1AC Grease 1 4 1 X Aero Shell 750 2 4 1 X Aero Shell 7A Grease 1 4 1 X Aerosafe 2300 4 1 4 X Aerosafe 2300W 4 1 4 X Aerozene 50 (50% Hydrazine 50% UDMH) 3 1 4 X Air, 1 1 1 X Air, 200 - 300° F 3 2 1 X Air, 300 - 400° F 4 4 1 X Air, 400 - 500° F 4 4 3 X Air,