Spin Injection and Transport in Organic Materials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spin Hall Angles in Solids Exhibiting a Giant Spin Hall Effect
Spin Hall Angles in Solids Exhibiting a Giant Spin Hall Effect BROWN Jamie Holber Thesis Advisor: Professor Gang Xiao Department of Physics Brown University A thesis submitted in fulfillment for the degree of Bachelor of Science in Physics April 2019 Abstract The Spin Hall Effect enables the conversion of charge current into spin current and provides a method for electrical control of the magnetization of a ferromagnet, enabling future applications in the transmission, storage, and control of information. The experimental values found for the Spin Hall Angle (ΘSH ), a way of quantifying the Spin Hall Effect in a nonmagnetic material, are studied in this thesis in regards to a variety of parameters including the atomic number, the fullness of the orbital, the spin diffusion length, thin film thickness, resistivity, temperature, and composition of alloys. We observed that the spin Hall angle dependence on atomic number, the fullness of the orbital, the spin diffusion length, and the resistivity aligned with the theory. The dependence on thickness and temperature was found to vary based on the material and more research is needed to determine larger trends. We found that alloys provide opportunities to create materials with large spin hall angles with lower resistivities. In particular, Au alloyed with other elements is a promising candidate. i Acknowledgements Thanks to my advisor, Professor Gang Xiao for his time and guidance working on my thesis. I would also like to thank the other members of my lab group who helped me and helped work on the project, Lijuan Qian, Wenzhe Chen, Guanyang He, Yiou Zhang, and Kang Wang. -

Introduction to Spin Hall Effect Spin-Dependent Lorentz Force Our
The spin Hall effect in a quantum gas M. C. Beeler*, R. A. Williams, K. Jimenez-Garcia, L. J. LeBlanc, A. R. Perry, I. B. Spielman Joint Quantum Institute, National Institute of Standards and Technology and Department of Physics, University of Maryland, Gaithersburg, MD 20899, USA Introduction to spin Hall effect Our system Spin currents Vector potential gradient • Spin Hall effect is separation of Vector potential is electron spins perpendicular to current proportional to flow coupling strength (intensity) • No external magnetic field needed – spin-orbit coupling drives effect Adjust equilibrium Current Flow Current position of BEC along • Effect is integral for spintronic devices Gaussian intensity and topological insulators gradient of Raman Color indicates predominant spin composition.1 beams 87 • Rb atoms in F = 1 ground state, confined in 90° cross-beam This is the first observation of the spin Hall effect in a optical dipole trap (ODT) Snapping off Raman cold atom system. beams gives electric • λ =790.21 nm Raman beams (Δω/2π = 15 MHz) couple mF = 0 field kick Spin-Dependent Lorentz Force and mF = -1 spin states, with large bias magnetic field along ez In intensity gradient, Lorentz Force • Adjustment of acousto-optic modulator frequency allows kick is spatially 퐹 = force dynamic control of BEC y-position by displacing one ODT beam dependent, shearing 퐹 = 푞 v × 퐵 q = charge the cloud after 2 v = velocity • No crystal structure, but system has spin-orbit coupling expansion • Cross product means that 퐵 = magnetic field particles always move • Atoms play the role of electrons, with spin coupling to linear Shear is opposite for perpendicular to velocity and 퐵 momentum along ex two pseudo-spins field. -

Observation of the Magnon Hall Effect
Observation of the magnon Hall effect [One-sentence summary] We have observed the Hall effect of magnons for the first time in terms of transverse thermal transport in a ferromagnetic insulator Lu2V2O7 with pyrochlore structure. Y. Onose,1,2 † T. Ideue,1 H. Katsura, 3 Y. Shiomi,1,4 N. Nagaosa,1, 4 and Y. Tokura1, 2, 4 1Department of Applied Physics, University of Tokyo, Tokyo 113-8656, Japan 2 Multiferroics Project, ERATO, Japan Science and Technology Agency (JST), c/o Department of Applied Physics, University of Tokyo, Tokyo 113-8656, Japan 3Kavli Institute for Theoretical Physics, University of California, Santa Barbara, CA 93106, USA 4Cross-Correlated Materials Research Group (CMRG) and Correlated Electron Research Group (CERG), Advanced Science Institute, RIKEN, Wako,351-0198, Japan † To whom correspondence should be addressed E-mail: [email protected] [abstract] The Hall effect usually occurs when the Lorentz force acts on a charge current in a conductor in the presence of perpendicular magnetic field. On the other hand, neutral quasi-particles such as phonons and spins can carry heat current and potentially show the Hall effect without resorting to the Lorentz force. We report experimental evidence for the anomalous thermal Hall effect caused by spin excitations (magnons) in an insulating ferromagnet with a pyrochlore lattice structure. Our theoretical analysis indicates that the propagation of the spin wave is influenced by the Dzyaloshinskii-Moriya spin-orbit interaction, which plays the role of the vector potential as in the intrinsic anomalous Hall effect in metallic ferromagnets. [text] Electronics based on the spin degree of freedom, (spintronics), holds promise for new developments beyond Si-based-technologies (1), and avoids the dissipation from Joule heating by replacing charge currents with currents of the magnetic moment (spin currents). -

Terahertz Spin Currents and Inverse Spin Hall Effect in Thin-Film Heterostructures Containing Complex Magnetic Compounds
Terahertz spin currents and inverse spin Hall effect in thin-film heterostructures containing complex magnetic compounds T. Seifert1, U. Martens2, S. Günther3, M. A. W. Schoen4, F. Radu5, X. Z. Chen6, I. Lucas7 , R. Ramos8, M. H. Aguirre9, P. A. Algarabel10, A. Anadón9, H. Körner4, J. Walowski2, C. Back4, M. R. Ibarra7, L. Morellón7, E. Saitoh8, M. Wolf1, C. Song6, K. Uchida11, M. Münzenberg2, I. Radu5, T. Kampfrath1,* 1Physical Chemistry, Fritz Haber Institute of the Max Planck Society, 14195 Berlin, Germany 2Institute of Physics, Ernst Moritz Arndt University, 17489 Greifswald, Germany 3Multifunctional Ferroic Materials Group, ETH Zürich, 8093 Zürich, Switzerland 4Institute for Experimental and Applied Physics, University of Regensburg, 93053 Regensburg, Germany 5Max-Born Institute for Nonlinear Optics and Short Pulse Spectroscopy, 12489 Berlin, Germany 6Key Laboratory of Advanced Materials, School of Materials Science and Engineering, Tsinghua University, 100084 Beijing, China 7Instituto de Nanociencia de Aragón, Universidad de Zaragoza, E-50018 Zaragoza, Spain 8WPI Advanced Institute for Materials Research, Tohoku University, 980-8577 Sendai, Japan 9Departamento de Física de la Materia Condensada, Universidad de Zaragoza, 50009 Zaragoza, Spain 10Instituto de Ciencia de Materiales de Aragón, Universidad de Zaragoza and Consejo Superior de Investigaciones Científicas, E- 50009 Zaragoza, Spain 11National Institute for Materials Science, 305-0047 Tsukuba, Japan * E-mail: [email protected] Abstract: Terahertz emission spectroscopy of ultrathin multilayers of magnetic and heavy metals has recently attracted much interest. This method not only provides fundamental insights into photoinduced spin transport and spin-orbit interaction at highest frequencies but has also paved the way to applications such as efficient and ultrabroadband emitters of terahertz electromagnetic radiation. -

Dynamical Measurements of the Spin Hall Angle Dissertation Zur
Dynamical measurements of the Spin Hall angle Dissertation zur Erlangung des Doktorgrades der Naturwissenschaften (Dr.rer.nat) der Fakultät für Physik der Universität Regensburg vorgelegt von Oleksandr Talalaevskyy aus Kiew März 2017 3 Contents Introduction 5 1. Magnetization Dynamics 9 1.1 Ferromagnetism…………………………………………………………………………………… 9 1.2 Landau-Lifshitz equation….…………………………………………………………..…………. 11 1.3 Consideration of the damping mechanism. Energy dissipation mechanisms…………………….. 13 1.4 FMR in thin films……………………………………………………………………………...…. 19 1.5 Magnetic properties of YIG……………………………………………………………………..... 21 2. Magnetostatic spin waves 23 2.1 Maxwell equations………………………………………………………………………………... 23 2.2 Magnetostatic approach and Walkers equation…………………………………………………... 24 2.3 Magnetostatic volume and surface waves………………………………………………………… 24 3. Spin pumping and spin Hall effect 29 3.1 Phenomenological explanation of spin pumping………………………………………………… 29 3.2 Theory of the spin pumping……………………………………………………………………… 29 3.3 Spin Hall effect phenomena……………………………………………………………………… 33 3.4 Skew scattering contribution……………………………………………………………………... 34 3.5 Side-jump contribution………………………………………………………………………….... 36 3.6 Intrinsic contribution………………………………………………………………………......…. 37 3.7 Experimental approach to SHE measurements…………………..……………………………….. 39 4 4. Experimental setups and sample preparation 41 4.1 FMR setup with coplanar waveguide……………………………………………………..……… 41 4.2 FMR setup with cavity………………….………………………………………………………... 43 4.3 Magneto-optical Kerr effect (MOKE)……………………………………………………………. -

Spin Hall Effect
Spin Hall Effect M. I. Dyakonov Université Montpellier II, CNRS, 34095 Montpellier, France 1. Introduction The Spin Hall Effect originates from the coupling of the charge and spin currents due to spin-orbit interaction. It was predicted in 1971 by Dyakonov and 1,2 Perel. Following the suggestion in Ref. 3, the first experiments in this domain were done by Fleisher's group at Ioffe Institute in Saint Petersburg, 4,5 providing the first observation of what is now called the Inverse Spin Hall Effect. As to the Spin Hall Effect itself, it had to wait for 33 years before it was experimentally observed7 by two groups in Santa Barbara (US) 6 and in Cambridge (UK). 7 These observations aroused considerable interest and triggered intense research, both experimental and theoretical, with hundreds of publications. The Spin Hall Effect consists in spin accumulation at the lateral boundaries of a current-carrying conductor, the directions of the spins being opposite at the opposing boundaries, see Fig. 1. For a cylindrical wire the spins wind around the surface. The boundary spin polarization is proportional to the current and changes sign when the direction of the current is reversed. j Figure 1. The Spin Hall Effect. An electrical current induces spin accumulation at the lateral boundaries of the sample. In a cylindrical wire the spins wind around the surface, like the lines of the magnetic field produced by the current. However the value of the spin polarization is much greater than the (usually negligible) equilibrium spin polarization in this magnetic field. 8 The term “Spin Hall Effect” was introduced by Hirsch in 1999. -

Spin Hall Effect for Microcavity Polaritons in Transition Metal
Spin Hall effect for polaritons in a TMDC monolayer embedded in a microcavity Oleg L. Berman1, Roman Ya. Kezerashvili1, and Yurii E. Lozovik2 1New York City College of Technology, The City University of New York 2Institute of Spectroscopy, Russian Academy of Sciences The work was supported by US Department of Defense under Grant No. W911NF1810433 and PSC CUNY under Grant No. 60599-00 48. OUTLINE • EXCITONS AND MICROCAVITY POLARITONS • SPIN HALL EFFECT • EXCITONS IN TMDCs MONOLAYERS • SPIN HALL EFFECT FOR POLARITONS IN A TMDC MONOLAYER EMBEDDED IN A MICROCAVITY • CONCLUSIONS Semiconductor microcavity structure Nature 443, 409 (2006). R. B. Balili, V. Hartwell, D. W. Snoke, L. Pfeiffer and K. West, Science 316, 1007 (2007). GaAs microcavities The spin Hall effect Relativistic Spin-Orbit Coupling • Relativistic effect: a particle in an electric field experiences an internal effective magnetic field in its moving frame E − + Beff ~ v E • Spin-Orbit coupling is the E coupling of spin with the internal effective magnetic field H ~ −S Beff v Spin-orbit coupling (relativistic effect) 1 Ingredients: - potential V(r) Produces E = − V (r) an electric field e E In the rest frame of an electron − - motion+ of an electron the electric field generates and effective magnetic field k B = − E eff cm - gives an effective interaction with the electron’s magnetic moment k E HSO = −μ Beff Beff Ordinary Hall effect [Hall 1879] B Lorentz force deflect like-charge particles _ _ _ _ _ _ _ _ _ _ _ FL + + + + + + + + + + + + + Ordinary: I Sign and density of V carriers; holes in SC Edwin H. -

Spin Conversion on the Nanoscale
PROGRESS ARTICLES PUBLISHED ONLINE: 10 JULY 2017 | DOI: 10.1038/NPHYS4192 Spin conversion on the nanoscale YoshiChika Otani1,2*, Masashi Shiraishi3, Akira Oiwa4, Eiji Saitoh5,6,7,8 and Shuichi Murakami9,10 Spins can act as mediators to interconvert electricity, light, sound, vibration and heat. Here, we give an overview of the recent advances in dierent sub-disciplines of spintronics that can be associated with the developing field of spin conversion, and discuss future prospects. pin conversion is a generic term for the phenomena associated scattering owing to spin–orbit coupling in non-ferromagnetic heavy with the interconversion between different physical entities— metals consisting of 4d and 5d transition metals, such as palladium, Selectricity, light, sound, vibration and heat—that are mediated tantalum, tungsten and platinum. In the reverse process, magnetic by spins (Fig.1). Most spin-conversion phenomena take place at the or spin Hall materials can also detect the spin accumulation, which nanoscale, in the regions near the interface of two diverse varieties is the central method of magneto-electric conversion from spin to of materials, such as magnets, non-magnets, semiconductors and charge information. insulators. In the above physical entities, electricity, light and Spin pumping, however, is a dynamic spin injection that heat represent electrons, photons and phonons, respectively, all relies on the dissipation of spin angular momentum—pure spin of which can transfer angular momentum through spins. Both currents from a resonantly precessing ferromagnetic moment sound and vibration—kinds of phonons in a broad sense—are are injected into an adjacent exchange-coupled non-magnetic mechanical motion, carrying mechanical angular momentum, material. -

Heat, Charge, and Spin Flow in Nanostructures for Information and Energy
Heat, charge, and spin flow in nanostructures for information and energy Barry Zink Department of Physics, University of Denver, Denver, CO 80208 A long-standing goal of spintronics is to identify faster, more energy-efficient nanoelectronic technologies by harnessing the spin degree of freedom in materials. More recently harnessing spin has also been envisioned as a possible route to new energy harvesting materials, for example by improving the efficiency of thermoelectric energy generation with thermomagnetic or spin transport effects. For all these applications, understanding the fundamental interactions of heat, charge, and spin in nanoscale materials is critical to continued progress. Accurate measurements of heat flow in these tiny systems presents a particular challenge, as reliable measurements of thermal effects in thin films and nanostructures require accurate generation and control of the thermal gradient applied to systems with tiny thermal mass. In this talk I will first overview the challenges of nanoscale thermal measurements, continue to describe our experimental solutions for these measurements, and finally present recent results on two very different systems: doped carbon nanotube/polymer hybrid thermoelectrics and nanoscale metallic non local spin valves. If time allows I will briefly highlight ongoing work on spin transport in magnetic insulators and spin Hall effects in metallic alloys. This work was carried out in collaboration with DU PhD students Alex Hojem, Devin Wesenberg, and Rachel Bennet; NREL collaborators including Azure Avery, Andrew Ferguson and Jeffery Blackburn; the DU Xin Fan group; and the CSU Mingzhong Wu group and is supported by the NSF (DMR and EECS). Phase-sensitive inductive detection of AC charge currents due to spin-pumping and spin-charge transduction in unpatterned Permalloy/Pt bilayers Andrew J. -

The Quantum Spin Hall Effect and Topological Insulators Xiao-Liang Qi, and Shou-Cheng Zhang
The quantum spin Hall effect and topological insulators Xiao-Liang Qi, and Shou-Cheng Zhang Citation: Physics Today 63, 1, 33 (2010); doi: 10.1063/1.3293411 View online: https://doi.org/10.1063/1.3293411 View Table of Contents: http://physicstoday.scitation.org/toc/pto/63/1 Published by the American Institute of Physics Articles you may be interested in Foundational theories in topological physics garner Nobel Prize Physics Today 69, 14 (2016); 10.1063/PT.3.3381 Two-dimensional van der Waals materials Physics Today 69, 38 (2016); 10.1063/PT.3.3297 Topological phases and quasiparticle braiding Physics Today 65, 38 (2012); 10.1063/PT.3.1641 Surprises from the spin Hall effect Physics Today 70, 38 (2017); 10.1063/PT.3.3625 Topological quantum computation Physics Today 59, 32 (2006); 10.1063/1.2337825 Fifty years of Anderson localization Physics Today 62, 24 (2009); 10.1063/1.3206091 The quantum spin Hall effect and feature topological insulators Xiao-Liang Qi and Shou-Cheng Zhang In topological insulators, spin–orbit coupling and time-reversal symmetry combine to form a novel state of matter predicted to have exotic physical properties. Xiao-Liang Qi is a research associate at the Stanford Institute for Materials and Energy Science and Shou-Cheng Zhang is a professor of physics at Stanford University in Stanford, California. In the quantum world, atoms and their electrons can crystals.6–8 QSH systems are insulating in the bulk—they have form many different states of matter, such as crystalline solids, an energy gap separating the valence and conduction bands— magnets, and superconductors. -

Spin-Torque and Spin-Hall Nano-Oscillators Tingsu Chen, Student Member, IEEE, Randy K
This work has been submitted to the IEEE for possible publication. Copyright may be transferred without notice, after which this version may no longer be available. Spin-Torque and Spin-Hall Nano-Oscillators Tingsu Chen, Student Member, IEEE, Randy K. Dumas, Member, IEEE, Anders Eklund, Student Member, IEEE, Pranaba K. Muduli, Member, IEEE, Afshin Houshang, Student Member, IEEE, Ahmad A. Awad, Member, IEEE, Philipp Dürrenfeld, Student Member, IEEE, B. Gunnar Malm, Senior Member, IEEE, Ana Rusu, Member, IEEE, and Johan Åkerman, Member, IEEE (Invited Paper) Abstract—This paper reviews the state of the art in were published at a time when magnetic thin film technol- spin-torque and spin Hall effect driven nano-oscillators. ogy had just become capable of beginning to implement After a brief introduction to the underlying physics, these concepts in earnest, with giant magnetoresistance the authors discuss different implementations of these (GMR) [7], [8] multilayers being prepared for hard drive oscillators, their functional properties in terms of fre- read-head production and the first high-quality magnetic quency range, output power, phase noise, and modu- tunnel junctions having been reported the year before [9], lation rates, and their inherent propensity for mutual synchronization. Finally, the potential for these oscil- [10]. It nevertheless took a few more years before the first lators in a wide range of applications, from microwave experimental demonstration of STT-driven magnetization signal sources and detectors to neuromorphic compu- precession was reported [11], followed by reports of STT- tation elements, is discussed together with the specific driven magnetic switching [12], [13]. electronic circuitry that has so far been designed to In 1999, Hirsch revisited [14] a different phenomenon, harness this potential. -
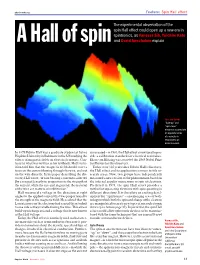
A Hall of Spinthe Experimental Observation of the Spin Hall Effect Could Open up a New Era in Spintronics, As Vanessa Sih, Yuich
physicsweb.org Feature: Spin Hall effect The experimental observation of the spin Hall effect could open up a new era in spintronics, as Vanessa Sih, Yuichiro Kato A Hall of spin and David Awschalom explain Ups and downs “Spin-up” and “spin-down” electrons accumulate on opposite sides of a sample in response to an electric current. In 1879 Edwin Hall was a graduate student at Johns is increased – in 1980, the Hall effect is now used to pro- Hopkins University in Baltimore in the US studying the vide a calibration standard for electrical resistance. effects of magnetic fields on electrical currents. Con- Klaus von Klitzing was awarded the 1985 Nobel Prize trary to what was written in his textbook, Hall’s intu- for Physics for this discovery. ition told him that the magnetic field should exert a Today, over 125 years since Edwin Hall’s discovery, force on the current flowing through the wire, and not the Hall effect and its applications remain fertile re- on the wire directly. In his paper describing the dis- search areas. Now, two groups have independently covery Hall wrote, “A wire bearing a current is affected measured a new version of the phenomenon based on [by a magnet] exactly in proportion to the strength of the internal angular momentum or spin of electrons. the current, while the size and, in general, the material Predicted in 1971, the spin Hall effect provides a of the wire are matters of indifference.” method of separating electrons with spins pointing in Hall measured a voltage in the direction at right different directions.