Cell Differentiation and Replication During Postnatal Development of the Murine First Molar
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Ono -- PTH-Pthrp Receptor Signaling in Osterix-Expressing Progenitors.Pdf
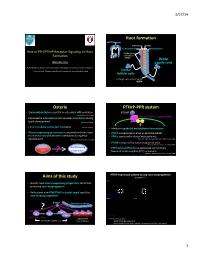
3/27/14 Root forma)on Cementum Dentin Cementoblast Odontoblast Role of PTH/PTHrP Receptor Signaling on Root Epithelial rests Formaon of Malassez (ERM) Dental Wanida Ono papilla cells AGE Orthodon;cs, Department of Developmental Biology, Harvard School of Dental Medicine Endocrine Unit, MassachuseLs General Hospital and Harvard Medical School Dental follicle cells Hertwig’s epithelial root sheath (HERS) Osterix PTHrP-PPR system • Transcripon factor essen;al to osteoblast differen;aon PTHrP (Nakashima K et al. 2002) PTH/PTHrP receptor (PPR) • Expressed in odontoblasts and alveolar osteoblasts during Gαs Gq tooth development (Chen S et al. 2009) • Controls cellular cementum formaon (Cao Z et al. 2012) • Mediates epithelial-mesenchymal interacons • Osterix-expressing precursors in the perichondrium move • PTHrP is expressed in enamel epithelia/HERS? to bone marrow and become osteoblasts during fetal • PPR is expressed in dental mesenchymes development (Maes C, Kronenberg HM et al. 2010) (Beck et al 1995; Lee Deeds and Segre 1995; Liu et al 1998) • PTHrP is required for tooth erup;on in mice (Philbrick WM, Karaplis AC et al. PNAS 1998) Osterix+ Root-forming • PPR haploinsufficiency is associated with primary cells progenitors failure of tooth erup;on (PFE) in humans ? (Decker E, Weber BH et al. Am J Hum Gen 2008) PTHrP expression paern during root morphogenesis Aims of this study PTHrPLacZ/+ x40 P7 P14 P49 • Iden;fy how osterix-expressing progenitors contribute to murine root morphogenesis • Understand how PTH/PTHrP receptor signal regulates root-forming progenitors PTHrP-LacZ x200 P7 x400 P14 ? PTHrP Epithelial root sheath PPR PTHrP-LacZ Osx+ progenitors Odontoblast PTHrP par;cipates in ……. -

Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes
Cells and Materials Volume 3 Number 2 Article 8 1993 Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes H. Lesot Institut de Biologie Médicale C. Begue-Kirn Institut de Biologie Médicale M. D. Kubler Institut de Biologie Médicale J. M. Meyer Institut de Biologie Médicale A. J. Smith Dental School, Birmingham See next page for additional authors Follow this and additional works at: https://digitalcommons.usu.edu/cellsandmaterials Part of the Biomedical Engineering and Bioengineering Commons Recommended Citation Lesot, H.; Begue-Kirn, C.; Kubler, M. D.; Meyer, J. M.; Smith, A. J.; Cassidy, N.; and Ruch, J. V. (1993) "Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes," Cells and Materials: Vol. 3 : No. 2 , Article 8. Available at: https://digitalcommons.usu.edu/cellsandmaterials/vol3/iss2/8 This Article is brought to you for free and open access by the Western Dairy Center at DigitalCommons@USU. It has been accepted for inclusion in Cells and Materials by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. Experimental Induction of Odontoblast Differentiation and Stimulation During Preparative Processes Authors H. Lesot, C. Begue-Kirn, M. D. Kubler, J. M. Meyer, A. J. Smith, N. Cassidy, and J. V. Ruch This article is available in Cells and Materials: https://digitalcommons.usu.edu/cellsandmaterials/vol3/iss2/8 Cells and Materials, Vol. 3, No. 2, 1993 (Pages201-217) 1051-6794/93$5. 00 +. 00 Scanning Microscopy International, Chicago (AMF O'Hare), IL 60666 USA EXPERIMENTAL INDUCTION OF ODONTOBLAST DIFFERENTIATION AND STIMULATION DURING REPARATIVE PROCESSES 1 1 1 2 2 1 H. -

Journal of Dental Research
Journal of Dental Research http://jdr.sagepub.com/ Cell Differentiation and Matrix Organization in Engineered Teeth A. Nait Lechguer, M.L. Couble, N. Labert, S. Kuchler-Bopp, L. Keller, H. Magloire, F. Bleicher and H. Lesot J DENT RES 2011 90: 583 originally published online 4 February 2011 DOI: 10.1177/0022034510391796 The online version of this article can be found at: http://jdr.sagepub.com/content/90/5/583 Published by: http://www.sagepublications.com On behalf of: International and American Associations for Dental Research Additional services and information for Journal of Dental Research can be found at: Email Alerts: http://jdr.sagepub.com/cgi/alerts Subscriptions: http://jdr.sagepub.com/subscriptions Reprints: http://www.sagepub.com/journalsReprints.nav Permissions: http://www.sagepub.com/journalsPermissions.nav >> Version of Record - Apr 13, 2011 OnlineFirst Version of Record - Feb 4, 2011 What is This? Downloaded from jdr.sagepub.com at Service Commun de la Documentation Université de Strasbourg on September 6, 2013 For personal use only. No other uses without permission. © 2011 International & American Associations for Dental Research RESEARCH REPORTS Biomaterials & Bioengineering A. Nait Lechguer1,2, M.L. Couble3,4, N. Labert3,4, S. Kuchler-Bopp1,2, Cell Differentiation and L. Keller1,2, H. Magloire3,4, F. Bleicher3,4, Matrix Organization in and H. Lesot1,2* Engineered Teeth 1INSERM UMR 977, Faculté de Médecine, 11, rue Humann, F-67085 Strasbourg, France; 2Dental School, University of Strasbourg, Strasbourg, France; 3Université de Lyon, Faculté d’Odontologie, Rue Guillaume Paradin, F-69372 Lyon Cedex 08, France; and 4IGFL, CNRS UMR 5242, Ecole Normale Supérieure, 46 Allée d’Italie, 69364, Lyon Cedex 08, France; *corresponding author, [email protected] J Dent Res 90(5):583-589, 2011 ABSTRACT InTRODuCTIOn Embryonic dental cells were used to check a series of criteria to be achieved for tooth engineering. -

Intrusion of Incisors to Facilitate Restoration: the Impact on the Periodontium
Note: This is a sample Eoster. Your EPoster does not need to use the same format style. For example your title slide does not need to have the title of your EPoster in a box surrounded with a pink border. Intrusion of Incisors to Facilitate Restoration: The Impact on the Periodontium Names of Investigators Date Background and Purpose This 60 year old male had severe attrition of his maxillary and mandibular incisors due to a protrusive bruxing habit. The patient’s restorative dentist could not restore the mandibular incisors without significant crown lengthening. However, with orthodontic intrusion of the incisors, the restorative dentist was able to restore these teeth without further incisal edge reduction, crown lengthening, or endodontic treatment. When teeth are intruded in adults, what is the impact on the periodontium? The purpose of this study was to determine the effect of adult incisor intrusion on the alveolar bone level and on root length. Materials and Methods We collected the orthodontic records of 43 consecutively treated adult patients (aged > 19 years) from four orthodontic practices. This project was approved by the IRB at our university. Records were selected based upon the following criteria: • incisor intrusion attempted to create interocclusal space for restorative treatment or correction of excessive anterior overbite • pre- and posttreatment periapical and cephalometric radiographs were available • no incisor extraction or restorative procedures affecting the cementoenamel junction during the treatment period pretreatment pretreatment Materials and Methods We used cephalometric and periapical radiographs to measure incisor intrusion. The radiographs were imported and the digital images were analyzed with Image J, a public-domain Java image processing program developed at the US National Institutes of Health. -

External Root Resorption of Young Premolar Teeth in Dentition With
10.5005/jp-journals-10015-1236 KapilaORIGINAL Arambawatta RESEARCH et al External Root Resorption of Young Premolar Teeth in Dentition with Crowding Kapila Arambawatta, Roshan Peiris, Dhammika Ihalagedara, Anushka Abeysundara, Deepthi Nanayakkara ABSTRACT least understood type of root resorption, characterized by its The present study was conducted to investigate the prevalence FHUYLFDOORFDWLRQDQGLQYDVLYHQDWXUH7KLVUHVRUSWLYHSURFHVV of external cervical resorption (ECR) in different tooth surfaces which leads to prRJUHVVLYHDQGXVXDOO\GHVWUXFWLYHORVVRI RIPD[LOODU\¿UVWSUHPRODUVLQD6UL/DQNDQSRSXODWLRQ tooth structure has been a source of interest to clinicians A sample of 59 (15 males, 44 females) permanent maxillary 2-5 ¿UVWSUHPRODUV DJHUDQJH\HDUV ZHUHXVHG7KHWHHWK DQGUHVHDUFKHUVIRURYHUDFHQWXU\ 7KHH[DFWFDXVHRI had been extracted for orthodontic reasons and were stored (&5LVSRRUO\XQGHUVWRRG$OWKRXJKWKHHWLRORJ\DQG LQIRUPDOLQ0RUSKRORJLFDOO\VRXQGWHHWKZHUHVHOHFWHG SDWKRJHQHVLVUHPDLQREVFXUHVHYHUDOSRWHQWLDOSUHGLVSRVLQJ IRUWKHVWXG\7KHWHHWKZHUHVWDLQHGZLWKFDUEROIXFKVLQ7KH IDFWRUVKDYHEHHQSXWIRUZDUGDQGRIWKHVHWKHLQWUDFRURQDO cervical regions of the stained teeth were observed under 10× 6 PDJQL¿FDWLRQV XVLQJ D GLVVHFWLQJ PLFURVFRSH 2O\PSXV 6= bleaching has been the most widely documented factor. In WRLGHQWLI\DQ\UHVRUSWLRQDUHDV7KHUHVRUSWLRQDUHDVSUHVHQW addition, dental trauma, orthodontic treatment, periodontal on buccal, lingual, mesial and distal aspects of all teeth were WUHDWPHQWVXUJHU\LQYROYLQJWKHFHPHQWRHQDPHOMXQFWLRQ UHFRUGHG DQGLGLRSDWKLFHWLRORJ\KDYHDOVREHHQGHVFULEHG2,7-11 -

The Cementoenamel Junction: Gap, Overlay and Edge to Edge Relationships
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 8-1996 The Cementoenamel Junction: Gap, Overlay and Edge to Edge Relationships Elizabeth A. Gilb University of Tennessee, Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Anthropology Commons Recommended Citation Gilb, Elizabeth A., "The Cementoenamel Junction: Gap, Overlay and Edge to Edge Relationships. " Master's Thesis, University of Tennessee, 1996. https://trace.tennessee.edu/utk_gradthes/4128 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Elizabeth A. Gilb entitled "The Cementoenamel Junction: Gap, Overlay and Edge to Edge Relationships." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Master of Arts, with a major in Anthropology. Murray Marks, Major Professor We have read this thesis and recommend its acceptance: Richard Jantz, William M. Bass, David Gerard Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) To the Graduate Council: I am submitting herewith a thesis written by Elizabeth A. Gilb entitled "The Cementoenamel Junction: Gap, Overlay and Edge to Edge Relationships." I have examined the finalcopy of this thesis forform and content and recommend that it be accepted in partial fulfillmentof the requirements forthe degree of Master of Arts, with a major in Anthropology. -

Dental Cementum Reviewed: Development, Structure, Composition, Regeneration and Potential Functions
Braz J Oral Sci. January/March 2005 - Vol.4 - Number 12 Dental cementum reviewed: development, structure, composition, regeneration and potential functions Patricia Furtado Gonçalves 1 Enilson Antonio Sallum 1 Abstract Antonio Wilson Sallum 1 This article reviews developmental and structural characteristics of Márcio Zaffalon Casati 1 cementum, a unique avascular mineralized tissue covering the root Sérgio de Toledo 1 surface that forms the interface between root dentin and periodontal Francisco Humberto Nociti Junior 1 ligament. Besides describing the types of cementum and 1 Dept. of Prosthodontics and Periodontics, cementogenesis, attention is given to recent advances in scientific Division of Periodontics, School of Dentistry understanding of the molecular and cellular aspects of the formation at Piracicaba - UNICAMP, Piracicaba, São and regeneration of cementum. The understanding of the mechanisms Paulo, Brazil. involved in the dynamic of this tissue should allow for the development of new treatment strategies concerning the approach of the root surface affected by periodontal disease and periodontal regeneration techniques. Received for publication: October 01, 2004 Key Words: Accepted: December 17, 2004 dental cementum, review Correspondence to: Francisco H. Nociti Jr. Av. Limeira 901 - Caixa Postal: 052 - CEP: 13414-903 - Piracicaba - S.P. - Brazil Tel: ++ 55 19 34125298 Fax: ++ 55 19 3412 5218 E-mail: [email protected] 651 Braz J Oral Sci. 4(12): 651-658 Dental cementum reviewed: development, structure, composition, regeneration and potential functions Introduction junction (Figure 1). The areas and location of acellular Cementum is an avascular mineralized tissue covering the afibrillar cementum vary from tooth to tooth and along the entire root surface. Due to its intermediary position, forming cementoenamel junction of the same tooth6-9. -

Basic Histology (23 Questions): Oral Histology (16 Questions
Board Question Breakdown (Anatomic Sciences section) The Anatomic Sciences portion of part I of the Dental Board exams consists of 100 test items. They are broken up into the following distribution: Gross Anatomy (50 questions): Head - 28 questions broken down in this fashion: - Oral cavity - 6 questions - Extraoral structures - 12 questions - Osteology - 6 questions - TMJ and muscles of mastication - 4 questions Neck - 5 questions Upper Limb - 3 questions Thoracic cavity - 5 questions Abdominopelvic cavity - 2 questions Neuroanatomy (CNS, ANS +) - 7 questions Basic Histology (23 questions): Ultrastructure (cell organelles) - 4 questions Basic tissues - 4 questions Bone, cartilage & joints - 3 questions Lymphatic & circulatory systems - 3 questions Endocrine system - 2 questions Respiratory system - 1 question Gastrointestinal system - 3 questions Genitouirinary systems - (reproductive & urinary) 2 questions Integument - 1 question Oral Histology (16 questions): Tooth & supporting structures - 9 questions Soft oral tissues (including dentin) - 5 questions Temporomandibular joint - 2 questions Developmental Biology (11 questions): Osteogenesis (bone formation) - 2 questions Tooth development, eruption & movement - 4 questions General embryology - 2 questions 2 National Board Part 1: Review questions for histology/oral histology (Answers follow at the end) 1. Normally most of the circulating white blood cells are a. basophilic leukocytes b. monocytes c. lymphocytes d. eosinophilic leukocytes e. neutrophilic leukocytes 2. Blood platelets are products of a. osteoclasts b. basophils c. red blood cells d. plasma cells e. megakaryocytes 3. Bacteria are frequently ingested by a. neutrophilic leukocytes b. basophilic leukocytes c. mast cells d. small lymphocytes e. fibrocytes 4. It is believed that worn out red cells are normally destroyed in the spleen by a. neutrophils b. -

The Role of Transforming Growth Factor Beta in Tertiary Dentinogenesis
The role of transforming growth factor beta in tertiary dentinogenesis Tetiana Haniastuti1, Phides Nunez2, and Ariadna A. Djais3 1Department of Oral Biology, Gadjah Mada University, Indonesia 2Division of Oral Ecology in Health and Infection, Niigata University, Japan 3Department of Oral Biology, University of Indonesia, Indonesia ABSTRACT The most visible repair response to pulp injury is the deposition of a tertiary dentin matrix over the dentinal tubules of the primary or secondary dentin. Tertiary dentin is distinguished as reactionary and reparative dentin, depending on the severity of the initiating response and the conditions under which the newly deposited dentin matrix was elaborated. Transforming growth factor beta (TGF-b) superfamily is a large group of growth factors that serve important roles in regulating cell growth, differentiation, and function. Members of this superfamily have been implicated in the repair process of the dental tissue after injury. Although numerous studies have proved that those bioactive molecules carry out an important role in the formation of tertiary dentin, comprehensive report regarding that phenomenon is not yet available. This review article aimed to summarize the role of TGF-b on tertiary dentinogenesis during the progression of a carious lesion. Key words: transforming growth factor beta, tertiary dentinogenesis, reactionary dentin, reparative dentin Correspondence: Tetiana Haniastuti, c/o: Bagian Biologi Oral, Fakultas Kedokteran Gigi Universitas Gadjah Mada. Jln. Denta no. 1, Sekip Utara Yogyakarta 55281, Indonesia. E-mail: [email protected] INTRODUCTION such as cell proliferation, differentiation, and matrix synthesis. Recent studies showed that this substance has a The complex structural composition of teeth provides significant role in the immune response7 and tissue repair8 hardness and durability as a barrier against bacterial of the dental pulp. -

Micro-CT Evaluation of Root and Canal Morphology of Mandibular First Premolars with Radicular Grooves
Brazilian Dental Journal (2017) 28(5): 597-603 ISSN 0103-6440 http://dx.doi.org/10.1590/0103-6440201601784 1Department of Restorative Dentistry, Micro-CT Evaluation of Root and School of Dentistry of Ribeirao Preto, USP – Universidade de São Canal Morphology of Mandibular First Paulo, Ribeirao Preto, SP, Brazil 2Department of Endodontics, Premolars with Radicular Grooves School of Dentistry, UNAERP - Universidade de Ribeirão Preto, Ribeirão Preto, SP, Brazil Correspondence: Manoel Damião de Sousa-Neto, Rua Célia de Oliveira 1 2 Emanuele Boschetti , Yara Terezinha Correa Silva-Sousa , Jardel Francisco Meirelles 350, 14024-070, Ribeirão Mazzi-Chaves1, Graziela Bianchi Leoni2, Marco Aurélio Versiani1, Jesus Djalma Preto, SP, Brasil. Tel: +55-16-9991- Pécora1, Paulo Cesar Saquy1, Manoel Damião de Sousa-Neto1 2696. e-mail: [email protected] The aim of this study was to evaluate morphological features of 70 single-rooted mandibular first premolars with radicular grooves (RG) using micro-CT technology. Teeth were scanned and evaluated regarding the morphology of the roots and root canals as well as length, depth and percentage frequency location of the RG. Volume, surface area and Structure Model Index (SMI) of the canals were measured for the full root length. Two-dimensional parameters and frequency of canal orifices were evaluated at 1, 2, and 3 mm levels from the apical foramen. The number of accessory canals, the dentinal thickness, and cross-sectional appearance of the canal at different root levels were also recorded. Expression of deep grooves was observed in 21.42% of the sample. Mean lengths of root and RG were 13.43 mm and 8.5 mm, respectively, while depth of the RG ranged from 0.75 to 1.13 mm. -

A Regenerative Endodontic Approach in Mature Ferret Teeth Using
in vivo 33 : 1143-1150 (2019) doi:10.21873/invivo.11584 A Regenerative Endodontic Approach in Mature Ferret Teeth Using Rodent Preameloblast-conditioned Medium CRISTINA BUCCHI 1,2 , ÁLVARO GIMENO-SANDIG 3, IVÁN VALDIVIA-GANDUR 4, CRISTINA MANZANARES-CÉSPEDES 1 and JOSEP MARIA DE ANTA 1 1Human Anatomy and Embryology Unit, Department of Pathology and Experimental Therapy, Faculty of Medicine and Health Sciences, Bellvitge Health Science Campus, University of Barcelona, Barcelona, Spain; 2Department of Integral Adult Dentistry, CICO Research Centre, University of La Frontera, Temuco, Chile; 3Biotherium Bellvitge Health Science Campus, Scientific and Technological Centers, University of Barcelona, Barcelona, Spain; 4Biomedical Department and Dentistry Department, University of Antofagasta, Antofagasta, Chile Abstract. Background: This study evaluated the effectiveness This therapy has been studied mainly for treating immature of a regenerative endodontic approach to regenerate the pulp necrotic teeth, as an attempt to complete the development of tissue in mature teeth of ferret. The presence of odontoblast- the fragile dentin walls and revitalize the tooth. like cells in the newly-formed tissue of teeth treated with or In clinical practice, most cases of pulp necrosis are found in without preameloblast-conditioned medium was evaluated mature teeth. However, studies oriented to regenerate or repair based on morphological criteria. Materials and Methods: pulp tissue of mature teeth are today scarce, most of them Twenty-four canines from six ferrets were treated. The pulp published in recent years (3-7). Currently, the most reliable was removed, and the apical foramen was enlarged. After option for the treatment of necrotic mature teeth is still root inducing the formation of a blood clot, a collagen sponge with canal treatment. -

Diagnosis Questions and Answers
1.0 DIAGNOSIS – 6 QUESTIONS 1. Where is the narrowest band of attached gingiva found? 1. Lingual surfaces of maxillary incisors and facial surfaces of maxillary first molars 2. Facial surfaces of mandibular second premolars and lingual of canines 3. Facial surfaces of mandibular canines and first premolars and lingual of mandibular incisors* 4. None of the above 2. All these types of tissue have keratinized epithelium EXCEPT 1. Hard palate 2. Gingival col* 3. Attached gingiva 4. Free gingiva 16. Which group of principal fibers of the periodontal ligament run perpendicular from the alveolar bone to the cementum and resist lateral forces? 1. Alveolar crest 2. Horizontal crest* 3. Oblique 4. Apical 5. Interradicular 33. The width of attached gingiva varies considerably with the greatest amount being present in the maxillary incisor region; the least amount is in the mandibular premolar region. 1. Both statements are TRUE* 39. The alveolar process forms and supports the sockets of the teeth and consists of two parts, the alveolar bone proper and the supporting alveolar bone; ostectomy is defined as removal of the alveolar bone proper. 1. Both statements are TRUE* 40. Which structure is the inner layer of cells of the junctional epithelium and attaches the gingiva to the tooth? 1. Mucogingival junction 2. Free gingival groove 3. Epithelial attachment * 4. Tonofilaments 1 49. All of the following are part of the marginal (free) gingiva EXCEPT: 1. Gingival margin 2. Free gingival groove 3. Mucogingival junction* 4. Interproximal gingiva 53. The collar-like band of stratified squamous epithelium 10-20 cells thick coronally and 2-3 cells thick apically, and .25 to 1.35 mm long is the: 1.