Cancer Facts & Figures 2015 Special Section
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genetic Markers in Lung Cancer Diagnosis: a Review
International Journal of Molecular Sciences Review Genetic Markers in Lung Cancer Diagnosis: A Review Katarzyna Wadowska 1 , Iwona Bil-Lula 1 , Łukasz Trembecki 2,3 and Mariola Sliwi´ ´nska-Mosso´n 1,* 1 Department of Medical Laboratory Diagnostics, Division of Clinical Chemistry and Laboratory Haematology, Wroclaw Medical University, 50-556 Wroclaw, Poland; [email protected] (K.W.); [email protected] (I.B.-L.) 2 Department of Radiation Oncology, Lower Silesian Oncology Center, 53-413 Wroclaw, Poland; [email protected] 3 Department of Oncology, Faculty of Medicine, Wroclaw Medical University, 53-413 Wroclaw, Poland * Correspondence: [email protected]; Tel.: +48-71-784-06-30 Received: 1 June 2020; Accepted: 25 June 2020; Published: 27 June 2020 Abstract: Lung cancer is the most often diagnosed cancer in the world and the most frequent cause of cancer death. The prognosis for lung cancer is relatively poor and 75% of patients are diagnosed at its advanced stage. The currently used diagnostic tools are not sensitive enough and do not enable diagnosis at the early stage of the disease. Therefore, searching for new methods of early and accurate diagnosis of lung cancer is crucial for its effective treatment. Lung cancer is the result of multistage carcinogenesis with gradually increasing genetic and epigenetic changes. Screening for the characteristic genetic markers could enable the diagnosis of lung cancer at its early stage. The aim of this review was the summarization of both the preclinical and clinical approaches in the genetic diagnostics of lung cancer. The advancement of molecular strategies and analytic platforms makes it possible to analyze the genome changes leading to cancer development—i.e., the potential biomarkers of lung cancer. -

DCIS): Pathological Features, Differential Diagnosis, Prognostic Factors and Specimen Evaluation
Modern Pathology (2010) 23, S8–S13 S8 & 2010 USCAP, Inc. All rights reserved 0893-3952/10 $32.00 Ductal carcinoma in situ (DCIS): pathological features, differential diagnosis, prognostic factors and specimen evaluation Sarah E Pinder Breast Research Pathology, Research Oncology, Division of Cancer Studies, King’s College London, Guy’s Hospital, London, UK Ductal carcinoma in situ (DCIS) is a heterogeneous, unicentric precursor of invasive breast cancer, which is frequently identified through mammographic breast screening programs. The lesion can cause particular difficulties for specimen handling in the laboratory and typically requires even more diligent macroscopic assessment and sampling than invasive disease. Pitfalls and tips for macroscopic handling, microscopic diagnosis and assessment, including determination of prognostic factors, such as cytonuclear grade, presence or absence of necrosis, size of the lesion and distance to margins are described. All should be routinely included in histopathology reports of this disease; in order not to omit these clinically relevant details, synoptic reports, such as that produced by the College of American Pathologists are recommended. No biomarkers have been convincingly shown, and validated, to predict the behavior of DCIS till date. Modern Pathology (2010) 23, S8–S13; doi:10.1038/modpathol.2010.40 Keywords: ductal carcinoma in situ (DCIS); breast cancer; histopathology; prognostic factors Ductal carcinoma in situ (DCIS) is a malignant, lesions, a good cosmetic result can be obtained by clonal proliferation of cells growing within the wide local excision. Recurrence of DCIS generally basement membrane-bound structures of the breast occurs at the site of previous excision and it is and with no evidence of invasion into surrounding therefore better regarded as residual disease, as stroma. -

PROPOSED REGULATION of the STATE BOARD of HEALTH LCB File No. R057-16
PROPOSED REGULATION OF THE STATE BOARD OF HEALTH LCB File No. R057-16 Section 1. Chapter 457 of NAC is hereby amended by adding thereto the following provision: 1. The Division may impose an administrative penalty of $5,000 against any person or organization who is responsible for reporting information on cancer who violates the provisions of NRS 457. 230 and 457.250. 2. The Division shall give notice in the manner set forth in NAC 439.345 before imposing any administrative penalty 3. Any person or organization upon whom the Division imposes an administrative penalty pursuant to this section may appeal the action pursuant to the procedures set forth in NAC 439.300 to 439. 395, inclusive. Section 2. NAC 457.010 is here by amended to read as follows: As used in NAC 457.010 to 457.150, inclusive, unless the context otherwise requires: 1. “Cancer” has the meaning ascribed to it in NRS 457.020. 2. “Division” means the Division of Public and Behavioral Health of the Department of Health and Human Services. 3. “Health care facility” has the meaning ascribed to it in NRS 457.020. 4. “[Malignant neoplasm” means a virulent or potentially virulent tumor, regardless of the tissue of origin. [4] “Medical laboratory” has the meaning ascribed to it in NRS 652.060. 5. “Neoplasm” means a virulent or potentially virulent tumor, regardless of the tissue of origin. 6. “[Physician] Provider of health care” means a [physician] provider of health care licensed pursuant to chapter [630 or 633] 629.031 of NRS. 7. “Registry” means the office in which the Chief Medical Officer conducts the program for reporting information on cancer and maintains records containing that information. -

Understanding Ductal Carcinoma in Situ (DCIS)
Understanding ductal carcinoma in situ (DCIS) and deciding about treatment Understanding ductal carcinoma in situ (DCIS) and deciding about treatment Developed by National Breast and Ovarian Cancer Centre Funded by the Australian Government Department of Health and Ageing Understanding ductal carcinoma in situ Contents Acknowledgements .........................................................................................2 How to use this resource ..............................................................................3 Introduction ...........................................................................................................4 Why do I need treatment for DCIS? .........................................................5 Surgery ......................................................................................................................7 Radiotherapy ......................................................................................................11 What is the risk of developing invasive breast cancer or Understanding ductal carcinoma in situ (DCIS) and deciding about treatment was prepared and produced by: DCIS after treatment? ....................................................................................12 National Breast and Ovarian Cancer Centre What follow-up will I need? .......................................................................17 Level 1 Suite 103/355 Crown Street Surry Hills NSW 2010 How can I get more emotional support? .........................................18 Locked Bag 3 -

TUMOR and STAGING DATA Primary Site Code
SECTION IV - TUMOR and STAGING DATA Primary Site Code NAACCR Version 11.1 field "Primary Site", Item 400, columns 291-294 It is unclear how the 2007 MP/H rules may alter rules for assigning the best Primary Site Formatted: Left Code to each primary. Continue to use the following rules until new rules are issued. Enter the code for the site of origin from the Topography section of ICD-O-3. [Note that ICD-O-2 code C14.1, laryngopharynx, should not be used for diagnoses made on or after January 1, 1995. "Laryngopharynx" became an equivalent term under C13.9 (hypopharynx, NOS) as of this diagnosis date. Code C14.1 is not an ICD-O-3 code.] Enter the site code that matches the narrative primary site indicated in the medical record, or the site code most appropriate for the case. Site codes are found in ICD-O-3's Numerical Lists - Topography section (pages 45-65) and in its Alphabetic Index (pages 105-218). In ICD-O-3 primary site codes consist of the letter "C" followed by two digits, a decimal point, and a third digit. "C" should be entered but the decimal point should not be entered. Example: The primary site is "cardia of stomach". Look this up in the Alphabetic Index of ICD-O-3 under "stomach" or "cardia", and the corresponding code "C16.0" is found. Enter C160. Most sites include a third digit of "8" to be used for single tumors that overlap the boundaries of two or more anatomically contiguous subsites and whose exact point of origin cannot be determined, unless the combination of sites is specifically indexed elsewhere. -

2018 SEER Solid Tumor Manual
4/6/2018 Eight Groups are Revised for 2018 Head & Neck Colon (includes rectosigmoid and rectum for cases diagnosed 1/1/2018 forward) 2018 SEER Lung (2018 Draft not yet available) Breast Solid Tumor Manual Kidney Urinary Sites (2018 Draft not yet available) 2018 KCR SPRING TRAINING Non‐malignant CNS (2018 Draft not yet available) Malignant CNS and Peripheral Nerves (2018 Draft not yet available) 2019 Changes for other two Groups Solid Tumor Rules What we will cover: ◦ Overview of General rules Cutaneous melanoma (minor revisions and draft available now) • Cutaneous melanoma site rules will be revised for 2019 implementation to incorporate ◦ New Head and Neck rules information from the new WHO 4th Ed Tumors of Skin scheduled to be released in 2018. ◦ New Colorectal rules ◦ New Breast rules Other sites (minor revisions and draft available now) ◦ New Kidney rules • Primary sites excluded are: •Rectosigmoid and rectum which are included in 2018 Colon rules. •Peripheral nerves which are included in 2018 Malignant Brain rules. •Other sites rules will be revised for 2019 implementation. The Solid Tumor Task Force has identified Remember: These are currently in draft form and may change slightly in the final version! the need to expand the rules to include GYN, soft tissue, thyroid and other site‐specific solid tumors. NEW! Code subtypes/variants when definitively described (with no modifiers) General Instructions Example: Well ‐differentiated neuroendocrine tumor 8240. Do not code a histology (*including subtypes or variants) when described as below: The 2018 solid tumor rules replace all previous MP/H rules, but they are effective for diagnoses on or after 1/1/2018. -

Surgical Management of Brain Tumors
SURGICAL MANAGEMENT OF BRAIN TUMORS JASSIN M. JOURIA, MD DR. JASSIN M. JOURIA IS A MEDICAL DOCTOR, PROFESSOR OF ACADEMIC MEDICINE, AND MEDICAL AUTHOR. HE GRADUATED FROM ROSS UNIVERSITY SCHOOL OF MEDICINE AND HAS COMPLETED HIS CLINICAL CLERKSHIP TRAINING IN VARIOUS TEACHING HOSPITALS THROUGHOUT NEW YORK, INCLUDING KING’S COUNTY HOSPITAL CENTER AND BROOKDALE MEDICAL CENTER, AMONG OTHERS. DR. JOURIA HAS PASSED ALL USMLE MEDICAL BOARD EXAMS, AND HAS SERVED AS A TEST PREP TUTOR AND INSTRUCTOR FOR KAPLAN. HE HAS DEVELOPED SEVERAL MEDICAL COURSES AND CURRICULA FOR A VARIETY OF EDUCATIONAL INSTITUTIONS. DR. JOURIA HAS ALSO SERVED ON MULTIPLE LEVELS IN THE ACADEMIC FIELD INCLUDING FACULTY MEMBER AND DEPARTMENT CHAIR. DR. JOURIA CONTINUES TO SERVES AS A SUBJECT MATTER EXPERT FOR SEVERAL CONTINUING EDUCATION ORGANIZATIONS COVERING MULTIPLE BASIC MEDICAL SCIENCES. HE HAS ALSO DEVELOPED SEVERAL CONTINUING MEDICAL EDUCATION COURSES COVERING VARIOUS TOPICS IN CLINICAL MEDICINE. RECENTLY, DR. JOURIA HAS BEEN CONTRACTED BY THE UNIVERSITY OF MIAMI/JACKSON MEMORIAL HOSPITAL’S DEPARTMENT OF SURGERY TO DEVELOP AN E- MODULE TRAINING SERIES FOR TRAUMA PATIENT MANAGEMENT. DR. JOURIA IS CURRENTLY AUTHORING AN ACADEMIC TEXTBOOK ON HUMAN ANATOMY & PHYSIOLOGY. Abstract The field of brain tumor research, diagnosis, and treatment is rapidly evolving. Over 120 types of brain tumors have been identified to date, and that number continues to increase. As the information available about brain tumors grows, so does the ability to target screening and therapies to provide patients with optimal outcomes. It is critical that health clinicians understand the surgical and treatment options in order to educate patients and to develop a care plan that has a positive outcome while respecting the patient's needs and desires. -

Full Text (PDF)
Published OnlineFirst April 1, 2014; DOI: 10.1158/1940-6207.CAPR-13-0362 Cancer Prevention Research Article Research Inhibition of the Transition of Ductal Carcinoma In Situ to Invasive Ductal Carcinoma by a Gemini Vitamin D Analog Joseph Wahler1, Jae Young So1, Yeoun Chan Kim1, Fang Liu1,2,3, Hubert Maehr1, Milan Uskokovic1, and Nanjoo Suh1,3 Abstract Ductal carcinoma in situ (DCIS) is a nonmalignant lesion of the breast with the potential to progress to invasive ductal carcinoma (IDC). The disappearance and breakdown of the myoepithelial cell layer and basement membrane in DCIS have been identified as major events in the development of breast cancer. The MCF10DCIS.com cell line is a well-established model, which recapitulates the progression of breast cancer from DCIS to IDC. We have previously reported that a novel Gemini vitamin D analog, 1a,25-dihydroxy- 20R-21(3-hydroxy-3-deuteromethyl-4,4,4-trideuterobutyl)-23-yne-26,27-hexafluoro-cholecalciferol (BXL0124) is a potent inhibitor of the growth of MCF10DCIS.com xenografted tumors without hypercal- cemic toxicity. In this study, we utilized the MCF10DCIS.com in vivo model to assess the effects of BXL0124 on breast cancer progression from weeks 1 to 4. Upon DCIS progression to IDC from weeks 3 to 4, tumors lost the myoepithelial cell layer and basement membrane as shown by immunofluorescence staining with smooth muscle actin and laminin 5, respectively. Administration of BXL0124 maintained the critical myoepithelial cell layer as well as basement membrane, and animals treated with BXL0124 showed a 43% reduction in tumor volume by week 4. -

Consensus Guideline on the Management of the Axilla in Patients with Invasive/In-Situ Breast Cancer
- Official Statement - Consensus Guideline on the Management of the Axilla in Patients With Invasive/In-Situ Breast Cancer Purpose To outline the management of the axilla for patients with invasive and in-situ breast cancer. Associated ASBrS Guidelines or Quality Measures 1. Performance and Practice Guidelines for Sentinel Lymph Node Biopsy in Breast Cancer Patients – Revised November 25, 2014 2. Performance and Practice Guidelines for Axillary Lymph Node Dissection in Breast Cancer Patients – Approved November 25, 2014 3. Quality Measure: Sentinel Lymph Node Biopsy for Invasive Breast Cancer – Approved November 4, 2010 4. Prior Position Statement: Management of the Axilla in Patients With Invasive Breast Cancer – Approved August 31, 2011 Methods A literature review inclusive of recent randomized controlled trials evaluating the use of sentinel lymph node surgery and axillary lymph node dissection for invasive and in-situ breast cancer as well as the pathologic review of sentinel lymph nodes and indications for axillary radiation was performed. This is not a complete systematic review but rather, a comprehensive review of recent relevant literature. A focused review of non-randomized controlled trials was then performed to develop consensus guidance on management of the axilla in scenarios where randomized controlled trials data is lacking. The ASBrS Research Committee developed a consensus document, which was reviewed and approved by the ASBrS Board of Directors. Summary of Data Reviewed Recommendations Based on Randomized Controlled -

EAU Guidelines on the Diagnosis and Treatment of Urothelial Carcinoma in Situ Adrian P.M
European Urology European Urology 48 (2005) 363–371 EAU Guidelines EAU Guidelines on the Diagnosis and Treatment of Urothelial Carcinoma in Situ Adrian P.M. van der Meijdena,*, Richard Sylvesterb, Willem Oosterlinckc, Eduardo Solsonad, Andreas Boehlee, Bernard Lobelf, Erkki Rintalag for the EAU Working Party on Non Muscle Invasive Bladder Cancer aDepartment of Urology, Jeroen Bosch Hospital, Nieuwstraat 34, 5211 NL ’s-Hertogenbosch, The Netherlands bEORTC Data Center, Brussels, Belgium cDepartment of Urology, University Hospital Ghent, Ghent, Belgium dDepartment of Urology, Instituto Valenciano de Oncologia, Valencia, Spain eDepartment of Urology, Helios Agnes Karll Hospital, Bad Schwartau, Germany fDepartment of Urology, Hopital Ponchaillou, Rennes, France gDepartment of Urology, Helsinki University Hospital, Helsinki, Finland Accepted 13 May 2005 Available online 13 June 2005 Abstract Objectives: On behalf of the European Association of Urology (EAU), guidelines for the diagnosis, therapy and follow-up of patients with urothelial carcinoma in situ (CIS) have been established. Method: The recommendations in these guidelines are based on a recent comprehensive overview and meta-analysis in which two panel members have been involved (RS and AVDM). A systematic literature search was conducted using Medline, the US Physicians’ Data Query (PDQ), the Cochrane Central Register of Controlled Trials, and reference lists in trial publications and review articles. Results: Recommendations are provided for the diagnosis, conservative and radical surgical treatment, and follow- up of patients with CIS. Levels of evidence are influenced by the lack of large randomized trials in the treatment of CIS. # 2005 Elsevier B.V. All rights reserved. Keywords: Bladder cancer; Carcinoma in situ; Bacillus Calmette-Guerin; Chemotherapy; Diagnosis; Treatment; Follow up 1. -

Carcinoma in Situ
JPAC 13-65 Joint UKBTS Professional Advisory Committee (1) Summary Sheet 1. Paper for the JPAC meeting on: 14 November 2013 2. Date submitted: 31 October 2013 3. Title (including version no.): Carcinoma in Situ 4. Author(s): Dr Sue Barnes, Chair of the SAC on Care and Selection of Donors 5. Brief summary: The DSG allows the acceptance of a very limited number of donors diagnosed, treated and cured and only non- clonal premalignant disease. BSQR 2005 allows acceptance of donors with ‘in situ cancer with complete recovery’. The WHO recommends acceptance if ‘in situ carcinoma (e.g. BCC) has been successfully treated. The CoE recommends the donor may be accepted immediately after successful removal and cure. We are thus recommending a change to the current DSG entry for Malignancy to allow a wider range of carcinomas in situ to be accepted once cured and to clarify the position re premalignant conditions. 6. Action required by the JPAC: Discuss and approve recommendation (What do you want JPAC to do in response to this paper?) e.g. endorse a specific recommendation advise where there is a choice of possible actions advise on priorities within the work plan provide a steer on policy 7. Any other relevant information: (1) Joint United Kingdom Blood Transfusion Services Professional Advisory Committee Page 1 of 5 JPAC 13-65 Carcinoma in situ Background Carcinoma in situ (CIS) is an early form of cancer that is defined by the absence of invasion of tumor cells into the surrounding tissue, usually before penetration through the basement membrane. -
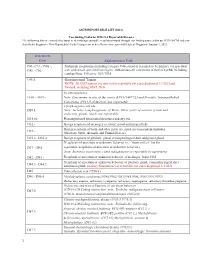
ASCR Reportable List ICD-10 Version
ASCR REPORTABLE LIST (2021) Casefinding Codes for ICD-O-3 Reportable Diseases The following lists are intended to assist in identifying reportable neoplasms found through casefinding sources that use ICD-10-CM codes to classify the diagnoses. New Reportable/ Code Changes are to be effective for cases with Date of Diagnosis January 1, 2021. ICD-10-CM Code Explanation of Code C00.- C43.-, C4A.-, Malignant neoplasms (excluding category C44), stated or resumed to be primary (of specified C45 - C96.- site) and certain specified histologies. Sebaceous cell carcinoma of skin of eyelid, including canthus Note: Effective 10/1/2018 C49.A- Gastrointestinal Tumors NOTE: All GIST tumors are now newly reportable for cases diagnosed 1/1/2021and forward, including GIST, NOS In-situ neoplasms D 00.- -D 09.- Note: Carcinoma in situ of the cervix (CIN 111-8077/2) and Prostatic lntraepithelial Carcinoma {PIN 111-8148/2) are not reportable Lymphangioma,any site D18.1 Note: Includes Lymphangiomas of Brain, Other parts of nervous system and endocrine glands, which are reportable D18.02 Hemangioma of intracranial structures and any site D32.- Benign neoplasm of meninges (cerebral, spinal and unspecified) Benign neoplasm of brain and other parts of central nervous system (includes D33.- Olfactory, Optic, Acoustic and Cranial Nerves) D35.2- D 35.4 Benign neoplasm of pituitary gland, craniopharyngeal duct and pineal gland Neoplasms of uncertain or unknown behavior (see "must collect" list for D37. - D41._ reportable neoplasms of uncertain or unknown behavior) Note: