Thunderlake 2009
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Northwest Territories Territoires Du Nord-Ouest British Columbia
122° 121° 120° 119° 118° 117° 116° 115° 114° 113° 112° 111° 110° 109° n a Northwest Territories i d i Cr r eighton L. T e 126 erritoires du Nord-Oues Th t M urston L. h t n r a i u d o i Bea F tty L. r Hi l l s e on n 60° M 12 6 a r Bistcho Lake e i 12 h Thabach 4 d a Tsu Tue 196G t m a i 126 x r K'I Tue 196D i C Nare 196A e S )*+,-35 125 Charles M s Andre 123 e w Lake 225 e k Jack h Li Deze 196C f k is a Lake h Point 214 t 125 L a f r i L d e s v F Thebathi 196 n i 1 e B 24 l istcho R a l r 2 y e a a Tthe Jere Gh L Lake 2 2 aili 196B h 13 H . 124 1 C Tsu K'Adhe L s t Snake L. t Tue 196F o St.Agnes L. P 1 121 2 Tultue Lake Hokedhe Tue 196E 3 Conibear L. Collin Cornwall L 0 ll Lake 223 2 Lake 224 a 122 1 w n r o C 119 Robertson L. Colin Lake 121 59° 120 30th Mountains r Bas Caribou e e L 118 v ine i 120 R e v Burstall L. a 119 l Mer S 117 ryweather L. 119 Wood A 118 Buffalo Na Wylie L. m tional b e 116 Up P 118 r per Hay R ark of R iver 212 Canada iv e r Meander 117 5 River Amber Rive 1 Peace r 211 1 Point 222 117 M Wentzel L. -

RURAL ECONOMY Ciecnmiiuationofsiishiaig Activity Uthern All
RURAL ECONOMY ciEcnmiIuationofsIishiaig Activity uthern All W Adamowicz, P. BoxaIl, D. Watson and T PLtcrs I I Project Report 92-01 PROJECT REPORT Departmnt of Rural [conom F It R \ ,r u1tur o A Socio-Economic Evaluation of Sportsfishing Activity in Southern Alberta W. Adamowicz, P. Boxall, D. Watson and T. Peters Project Report 92-01 The authors are Associate Professor, Department of Rural Economy, University of Alberta, Edmonton; Forest Economist, Forestry Canada, Edmonton; Research Associate, Department of Rural Economy, University of Alberta, Edmonton and Research Associate, Department of Rural Economy, University of Alberta, Edmonton. A Socio-Economic Evaluation of Sportsfishing Activity in Southern Alberta Interim Project Report INTROI)UCTION Recreational fishing is one of the most important recreational activities in Alberta. The report on Sports Fishing in Alberta, 1985, states that over 340,000 angling licences were purchased in the province and the total population of anglers exceeded 430,000. Approximately 5.4 million angler days were spent in Alberta and over $130 million was spent on fishing related activities. Clearly, sportsfishing is an important recreational activity and the fishery resource is the source of significant social benefits. A National Angler Survey is conducted every five years. However, the results of this survey are broad and aggregate in nature insofar that they do not address issues about specific sites. It is the purpose of this study to examine in detail the characteristics of anglers, and angling site choices, in the Southern region of Alberta. Fish and Wildlife agencies have collected considerable amounts of bio-physical information on fish habitat, water quality, biology and ecology. -

Brazeau Subwatershed
5.2 BRAZEAU SUBWATERSHED The Brazeau Subwatershed encompasses a biologically diverse area within parts of the Rocky Mountain and Foothills natural regions. The Subwatershed covers 689,198 hectares of land and includes 18,460 hectares of lakes, rivers, reservoirs and icefields. The Brazeau is in the municipal boundaries of Clearwater, Yellowhead and Brazeau Counties. The 5,000 hectare Brazeau Canyon Wildland Provincial Park, along with the 1,030 hectare Marshybank Ecological reserve, established in 1987, lie in the Brazeau Subwatershed. About 16.4% of the Brazeau Subwatershed lies within Banff and Jasper National Parks. The Subwatershed is sparsely populated, but includes the First Nation O’Chiese 203 and Sunchild 202 reserves. Recreation activities include trail riding, hiking, camping, hunting, fishing, and canoeing/kayaking. Many of the indicators described below are referenced from the “Brazeau Hydrological Overview” map locat- ed in the adjacent map pocket, or as a separate Adobe Acrobat file on the CD-ROM. 5.2.1 Land Use Changes in land use patterns reflect major trends in development. Land use changes and subsequent changes in land use practices may impact both the quantity and quality of water in the Subwatershed and in the North Saskatchewan Watershed. Five metrics are used to indicate changes in land use and land use practices: riparian health, linear development, land use, livestock density, and wetland inventory. 5.2.1.1 Riparian Health 55 The health of the riparian area around water bodies and along rivers and streams is an indicator of the overall health of a watershed and can reflect changes in land use and management practices. -
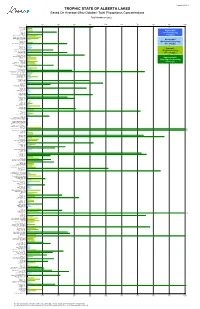
Trophic State of Alberta Lakes Based on Average Total Phosphorus
Created Feb 2013 TROPHIC STATE OF ALBERTA LAKES Based On Average (May-October) Total Phosphorus Concentrations Total Phosphorus (µg/L) 0 100 200 300 400 500 600 700 800 900 1000 * Adamson Lake Alix Lake * Amisk Lake * Angling Lake Oligotrophic * ‡ Antler Lake Arm Lake (Low Productivity) * Astotin Lake (<10 µg/L) * ‡ Athabasca (Lake) - Off Delta Baptiste Lake - North Basin Baptiste Lake - South Basin * ‡ Bare Creek Res. Mesotrophic * ‡ Barrier Lake ‡ Battle Lake (Moderate Productivity) * † Battle River Res. (Forestburg) (10 - 35 µg/L) Beartrap Lake Beauvais Lake Beaver Lake * Bellevue Lake Eutrophic * † Big Lake - East Basin * † Big Lake - West Basin (High Productivity) * Blackfalds Lake (35 - 100 µg/L) * † Blackmud Lake * ‡ Blood Indian Res. Bluet (South Garnier Lake) ‡ Bonnie Lake Hypereutrophic † Borden Lake * ‡ Bourque Lake (Very High Productivity) ‡ Buck Lake (>100 µg/L) Buffalo Lake - Main Basin Buffalo Lake - Secondary Bay * † Buffalo Lake (By Boyle) † Burntstick Lake Calling Lake * † Capt Eyre Lake † Cardinal Lake * ‡ Carolside Res. - Berry Creek Res. † Chain Lakes Res. - North Basin † Chain Lakes Res.- South Basin Chestermere Lake * † Chickakoo Lake * † Chickenhill Lake * Chin Coulee Res. * Clairmont Lake Clear (Barns) Lake Clear Lake ‡ Coal Lake * ‡ Cold Lake - English Bay ‡ Cold Lake - West Side ‡ Cooking Lake † Cow Lake * Crawling Valley Res. Crimson Lake Crowsnest Lake * † Cutbank Lake Dillberry Lake * Driedmeat Lake ‡ Eagle Lake ‡ Elbow Lake Elkwater Lake Ethel Lake * Fawcett Lake * † Fickle Lake * † Figure Eight Lake * Fishing Lake * Flyingshot Lake * Fork Lake * ‡ Fox Lake Res. Frog Lake † Garner Lake Garnier Lake (North) * George Lake * † Ghost Res. - Inside Bay * † Ghost Res. - Inside Breakwater ‡ Ghost Res. - Near Cochrane * Gleniffer Lake (Dickson Res.) * † Glenmore Res. -

Western Grebe Surveys in Alberta 2016
WESTERN GREBE SURVEYS IN ALBERTA 2016 The western grebe has been listed as a Threatened species in Alberta. A recent data compilation shows that there are approximately 250 lakes that have supported western grebes in Alberta. However, information for most lakes is poor and outdate d. Total counts on lakes are rare, breeding status is uncertain, and the location and extent of breeding habitat (emergent vegetation, usually bulrush) is usually unknown. We are seeking your help in gathering more information on western grebe populations in Alberta. If you visit any of the lakes listed below, or know anyone that does, we would appreciate as much detail as you can collect on the presence of western grebes and their habitat. Let us know in advance (if possible) if you are planning on going to any lakes, and when you do, e-mail details of your observations to [email protected]. SURVEY METHODS: Visit a lake between 1 May and 31 August with spotting scope or good binoculars. Surveys can be done from a boat, or vantage point(s) from shore. Report names of surveyors, dates, number of adults seen, and report on the approximate percentage of the lake area that this number represents. Record presence of young birds or nesting colonies, and provide any additional information on presence/location of likely breeding habitat, specific parts of the lake observed, observed threats to birds or habitat (boat traffic, shoreline clearing, pollution, etc.). Please report on findings even if no birds were seen. Lakes on the following page that are flagged with an asterisk (*) were not visited in 2015, and are priority for survey in 2016. -

Alberta Cyanobacteria Beach Monitoring 2010-2013
Alberta Health, Health Protection Alberta Health ALBERTA CYANOBACTERIA BEACH MONITORING 2010–2013 September 2014 Alberta Health, Health Protection Branch Alberta Cyanobacteria Beach Monitoring 2010–2013 September 2014 For more information contact: Health Protection Branch Alberta Health P.O. Box 1360, Station Main Edmonton, Alberta, T5J 1S6 Telephone: 1-780-427-1470 ISBN: 978-1-4601-1922-8 (PDF) 2014 Government of Alberta Alberta Health, Health Protection Branch Alberta Cyanobacteria Beach Monitoring 2010–2013 September 2014 EXECUTIVE SUMMARY Harmful blue-green algae (toxic cyanobacteria) blooms in surface water are prevalent in Alberta. The presence of blue-green algae in recreational water causes unpleasant aesthetics. Exposure to some toxin-producing blue-green algae may pose potential health risks to public. There have been increased public awareness and health concerns as a result of increased research over the past 20 years, recent monitoring efforts, as well as the general public becoming educated on the matter. In 2010 and 2011, Alberta Health Services initiated a cyanobacteria monitoring program for shallow water adjacent to beaches and issued public health advisories based on visual inspection. The findings revealed that microcystins (MCYSTs), one group of toxins produced by cyanobacteria, dominate in Alberta’s lakes and reservoirs. In order to inform the residents to safely use public beaches and implement better public health management, Alberta Health and Alberta Health Services along with other governmental departments and public health laboratories conducted the program of Alberta Cyanobacteria Beach Monitoring for Public Health in 2012 and 2013. The objectives of this program are to : 1. establish and maintain an integrated, participatory process for responding to and managing public health issues relating to harmful blue-green algae blooms, 2. -
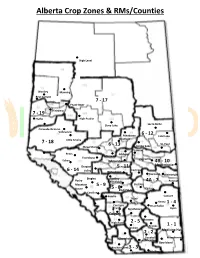
Alberta Crop Zones & Rms/Counties
Alberta Crop Zones & RMs/Counties High Level Worsley Manning Bear Canyon 7 - 17 Peace River Fairview 7 - 19 Hythe High Prairie Slave Lake Lac la Biche Grande Prairie Valleyview 6 - 12 Athabasca Cold Lake Westlock 7 - 18 Little Smoky 6 - 13 St. Paul Mayerthorpe Smoky Lake Lamont Peers Gibbons Onoway Evansburg Vegreville Edson Edmonton 4B - 10 Lloydminster Drayton 5 - 11 11 6 - 14 Valley Camrose Wetaskiwin Daysland Wainwright Bingley Rimbey Rocky Blackfalds 4A - 7 Provost Mountain 5 - 9 Stettler 5 - 8 Castor House Red Deer Caroline Sundre Consort Three Hills Hanna 1 - 4 Carstairs Drumheller 3 - 6 Strathmore Oyen Calgary Bassano High River 2 - 5 Brooks 1 - 1 Vulcan 2 - 2 Medicine Hat Claresholm Vauxhall Manyberries Lethbridge Bow Island Pincher Creek 3 - 3 Cardston Milk River Cities & Towns in Each Alberta Crop Zone 1 - 1 1 – 4 2 – 2 Pakowki Lucky Strike Aden Pashley Acadia Valley Albion Ridge Pendant Mallow Alderson Altario Allerston d'Oreille Masinasin Altorado Arneson Armelgra Pinhorn Matzhiwin Birdsholm Atlee Askow Pivot Maybutt Bow Island Benton Bantry Ralston McNab Bow River Benton Station Barnwell Ranchville Milk River Bowell Bindloss Barons Redcliff Millicent Bowmanton Buffalo Bassano Ronalane Monarch Bullshead Cavendish Bow City Rosebeg New Dayton Burdett Cereal Brooks Roytal Nobleford Cecil Cessford Broxburn Schuler Patricia Comrey Chinook Cassils Seven Persons Picture Butte Cressday Compeer Chin Skiff Piyami Dennis Consort Coaldale Stornham Princess Desert Blume Dorothy Coalhurst Suffield Purple Springs Dunmore Dowling Conrad Thelma Rainier Eagle Butte Drumheller Countess Tothill Raymond Etzikom Empress Coutts Vale Retlaw Florann Esther Craddock Veinerville Rolling Hills Foremost Excel Cranford Walsh Rosemary Gahern Finnegan Denhart Whitla Scandia Goddard Fleet Diamond City Wild Horse Shaughnessy Granlea Hanna Duchess Winnifred St. -

Occasional Paper: Archaeology in Alberta 1987
ARCHAEOLOGY IN ALBERTA, 1987 Compiled by Martin Magne Archaeological Survey of Alberta Occasional Paper No. 32 Prepared by: Published by: Archaeological Survey Alberta Culture and Multiculturalism of Alberta Historical Resources Division OCCASIONAL PAPERS Papers for publication in this series of monographs are produced by or for the five branches of the Historical Resources Division of Alberta Culture and Multiculturalism: the Provincial Archives of Alberta, the Provincial Museum of Alberta, the Historic Sites Service, the Archaeological Survey of Alberta, and the Tyrrell Museum of Palaeontology. Those persons or institutions interested in particular subjects may obtain pUblication lists from the appropriate branches. All publications produced by the Archaeological Survey of Alberta are distributed free of charge to the public. Requests for list of available pUblications and orders for specific papers should be addressed to: Occasional Papers Archaeological Survey of Alberta 8820 - 112 Street Edmonton, Alberta T6G 2P8 Phone (403) 431-2300 The Archaeological Survey of Alberta Occasional Papers are intended primarily for interested specialists rather than as popular publications for general readers. The Archaeological Survey encourages authors to submit manuscripts for publication on topics of Alberta archaeology. Editorial policy requires full length monographs to be subjected to peer review process. Papers pUblished in the Annual Review of Alberta Archaeology and edited thematic volumes are subject to the discretion of the series editor. To maintain a free distribution of Occasional Papers, production costs are minimized. iii TABLE OF CONTENTS List of Tables •• vii List of Figures ix Archaeology in Alberta, 1987 John W. Ives ••••• The 1987 Field Season at Head-Smashed-In Buffalo Jump: An Interim Report Jack Brink and Bob Dawe • 9 The Strathcona Site (FjPi-29): 1987 Season Preliminary Report Brian Kooyman ••• 19 An Archaeological Survey in the Upper North Saskatchewan River Valley Brian M. -

Visitation Statistics Provincial Parks & Recreation Areas 2002/03 Fiscal Year
Visitation Statistics Provincial Parks & Recreation Areas 2002/03 Fiscal Year prepared by Alberta Community Development Parks and Protected Areas Division Policy and Program Coordination Branch Visitation Statistics Provincial Parks & Recreation Areas 2002/03 Fiscal Year prepared by Alberta Community Development Parks and Protected Areas Division Policy and Program Coordination Branch Foreword The ‘Visitation Statistics, 2002/03 Fiscal Year’ report, In addition to visitation, this report provides information covering the period April 1, 2002 to March 31, 2003, is covering facilities and services for selected sites. To put the published annually to supply selected information on province in perspective, maps (see Appendix) are also provincial parks and recreation areas. This publication included. Maps 1 and 2 are for Southern and Northern Alberta provides data on the following aspects of park visitation: respectively. They show the location of sites reporting visitation statistics. Additional information on the provincial Camping Use Statistics parks and provincial recreation areas can be obtained from the Based on camping permit sales analysis, and / or reasonable following ‘Web Site’ : estimates of camping use and camper surveys. (http://www.cd.gov.ab.ca/enjoying_alberta/parks/) Day Use Statistics Inquiries regarding the statistics reported in this document or a Collected through Automatic Traffic Counter readings and further explanation of the methodology are welcomed, and traffic surveys. should be directed to the Section Head, Research Assessment Section at (780) 427-7009. Group Camping Use Statistics Based on group use permit sales analysis. Fixed Roof Accommodation Use Statistics Kananaskis Country - number of guests reported. Visitation statistics are reported both at the park and ‘area’ level (i.e., individual campgrounds, day use areas, group use areas, fixed roof accommodation facilities). -
WNV-Cases-Sept-21-20
Andrew Lake Beatty L. Charles Lake Potts L. Bistcho Lake (!35 Cornwall Lake Colin L. Thultue Lake Wood Buffalo Wylie L. I.D. No. 24 Wood Buffalo Wentzel Lake Margaret Lake Lake Athabasca Hay L. Zama Lake Baril Lake Mackenzie County Mamawi Lake Lake Claire Hilda L. Rainbow Lake (!58 (!88 High Level Richardson Lake Welstead Lake County of Northern Lights Wadlin Lake Regional Municipality of Wood Buffalo McClelland Lake Namur L. NOTIKEWIN Bison Lake Manning Clear Hills County Fort McMurray (!69 Peerless Lake Northern Sunrise County Vandersteene Lake Gordon Lake Graham Lake GREGOIRE LAKE Gipsy Lake Gregoire L. 64 (! Garson Lake Lubicon L. M.D. of Opportunity No. 17 Hines Creek Cardinal L. Peace River QUEENGrimshaw ELIZABETH M.D. of Peace No. 135Berwyn Fairview Muskwa Lake (!64A Nampa M.D. of Fairview No. 136 North Wabasca L. Bohn Lake DUNVEGAN Saddle Hills County MOONSHINE LAKE South Wabasca Lake Utikuma Lake M.D. of Spirit River No. 133 Sandy L. Spirit River Rycroft Birch Hills County Pelican L. Girouxville Nipisi Lake Kimiwan Lake (!49 Donnelly Falher McLennan Christina Lake M.D. of Smoky River No. 130Winagami L. WINAGAMI LAKE Winefred Lake (!63 HILLIARDS BAY 59 2A (! (! High Prairie LESSER SLAVE LAKE Wiau Lake Hythe Lesser Slave Lake Sexsmith M.D. of Lesser Slave River No. 124 Kinuso County of Grande Prairie No. 1 Bear L. Slave Lake M.D. of Big Lakes Fawcett Lake Lac La Biche County Beaverlodge !43 SASKATOON ISLAND ( (!43 Calling Lake Wembley GRANDE PRAIRIE CALLING LAKE YOUNGS POINT Snipe L. I.D. No. 349 OBRIEN Sturgeon L. -
´ DRAFT TEMPLATE Alberta Road Testing Regions
I.R. Andrew Bayonet 6G Lake Lake Spawn 19 Lake Creighton L s rcredi M ake Esk L. I.R. e Me ay e . k I.R L. k Lake 196A ulip a T a S D L 6 L Thurston Bu la 19 L. ff v I.R. Lake alo e 25 R 2 . Sass s Lake e d d Arch l n r n n a a Ashto s a . L h te Vermilion Kilome l i I.R. I.R. e Rat h C L. Lake I.R. L B La W Lake 196 196C eatty L. ke 196B Pans Potts Cherry L. Lake R Lake McLelland i Leggo ck B v Whaleba istcho Lake e L. ke eekes Dickins L. La W r Lake Lake WOOD I.R. St. 196F Agnes aup yers R M Lake r Lake Lake e I.R. v Arrowhead i 196E Alexander R Lake L. I.R. I.R. I.R. Pert L. Upland Daly Lake 223 214 ake all 213 L Cornw WALL LAKES Snake I.R.COLIN-CORN Lake Lake WILDLAND 4 ARK lin Thultue Barbant 22 PROVINCIAL P Co L. Lake L. Conibear Cockscomb Woodman ake Lake L. Hooker L. L Roderick Lake McNeil Pine Lake Peters Lake Lake W e ally Lan e E ake Bocquen L. lsa L Disappointment L. Robertson Lake Kennedy Lake . Jackfish L Turtle L. Bowhay Lake Lakes L. FALO BUF Burstall LA BUTTE CREEK Wylie P. PARK Lake Pierre WILDLAND L. y Lake in a r Darw . H Isidore e Lake Bryant L Lynx v Lake e i e L. -
2004 Municipal Codes
LOCAL GOVERNMENT SERVICES DIVISION MUNICIPAL SERVICES BRANCH Updated January 2004 2004 MUNICIPAL CODES 17th Floor Commerce Place 10155 - 102 Street Edmonton, Alberta T5J 4L4 Internet: http://www.gov.ab.ca/ma/ms/ Phone: (780) 427-7495 Fax: (780) 422-9133 E-mail: [email protected] 2004 MUNICIPAL CHANGES STATUS CHANGES: NAME CHANGES: AMALGAMATED: FORMATIONS: DISSOLVED: 0223 - Village of Mirror (effective January 1, 2004) to Lacombe County. CODE NUMBERS RESERVED: 0522 - Metis Settlements General Council 0524 - R.M. of Brittania (Sask.) 0462 - Townsite of Redwood Meadows STATUS CODES: 01 - Cities (15)* 15 - Hamlet & Urban Services Areas 09 - Specialized Municipalities (4) 20 - Service Commissions 06 - Municipal Districts (64) 25 - First Nations 02 - Towns (110) 26 - Indian Reserves 03 - Villages (102) 50 - Local Government Associations 04 - Summer Villages (51) 60 - Disaster Services 07 - Improvement Districts (7) 70 - Regional Health Authorities 08 - Special Areas (3) 98 - Reserved Codes 11 - Metis Settlements 99 - Dissolved * (Includes Lloydminster) January 2004 Page 1 CITIES (Status Code 01) CODE CITIES (Status Code 01) CODE NO. NO. Airdrie 0003 Lethbridge 0203 Calgary 0046 Lloydminster* 0206 Camrose 0048 Medicine Hat 0217 Cold Lake 0525 Red Deer 0262 Edmonton 0098 Spruce Grove 0291 Fort Saskatchewan 0117 St. Albert 0292 Grande Prairie 0132 Wetaskiwin 0347 Leduc 0200 *Alberta only SPECIALIZED MUNICIPALITY CODE SPECIALIZED MUNICIPALITY CODE (Status Code 09) NO. (Status Code 09) NO. Jasper, Municipality of 0418 Reg Mun of Wood Buffalo 0508 Mackenzie No. 23, M.D. of 0505 Strathcona County 0302 MUNICIPAL DISTRICTS CODE MUNICIPAL DISTRICTS CODE (Status Code 06) NO. (Status Code 06) NO. Acadia No. 34, M.D.