And Sex-Specific Predation on Dung Flies by Amphibian and Arthropod
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
PROCEEDINGS of the HAWAIIAN ENTOMOLOGICAL SOCIETY for 1973
PROCEEDINGS of the HAWAIIAN ENTOMOLOGICAL SOCIETY for 1973 VOL. XXII NO. 1 August 1975 Information for Contributors Manuscripts for publication, proof, and other editorial matters should be addressed to: Editor: Hawaiian Entomological Society c/o Department of Entomology University of Hawaii 2500 Dole Street, Honolulu, Hawaii 96822 Manuscripts should not exceed 40 typewritten pages, including illustrations (approx imately 20 printed pages) . Longer manuscripts may be rejected on the basis of length, or be subject to additional page charges. Typing—Manuscripts must be typewritten on one side of white bond paper, 8l/£ x 11 inches. Double space all text, including tables, footnotes, and reference lists. Margins should be a minimum of one inch. Underscore only where italics are intended in body of text, not in headings. Geographical names, authors names, and names of plants and animals should be spelled out in full. Except for the first time they are used, scientific names of organisms may be abbreviated by using the first letter of the generic name plus the full specific name. Submit original typescript and one copy. Pages should be numbered consecutively. Place footnotes at the bottom of the manuscript page on which they appear, with a dividing line. Place tables separately, not more than one table per manuscript page, at end of manuscript. Make a circled notation in margin of manuscript at approximate location where placement of a table is desired. Use only horizontal lines in tables. Illustrations—Illustrations should be planned to lit the type page of 4i/, x 7 inches, with appropriate space allowed for captions. Number all figures consecutively with Arabic numerals. -

The Nestling Diet of Svalbard Snow Buntings Identified by DNA Metabarcoding
Faculty of Biosciences, Fisheries and Economics, Department of Arctic and Marine Biology The nestling diet of Svalbard snow buntings identified by DNA metabarcoding — Christian Stolz BIO-3950 Master thesis in Biology, Northern Populations and Ecosystems, May 2019 Faculty of Biosciences, Fisheries and Economics, Department of Arctic and Marine Biology The nestling diet of Svalbard snow buntings identified by DNA metabarcoding Christian Stolz, UiT The Arctic University of Norway, Tromsø, Norway and The University Centre in Svalbard (UNIS), Longyearbyen, Norway BIO-3950 Master Thesis in Biology, Northern Populations and Ecosystems, May 2018 Supervisors: Frode Fossøy, Norwegian Institute for Nature Research (NINA), Trondheim, Norway Øystein Varpe, The University Centre in Svalbard (UNIS), Longyearbyen, Norway Rolf Anker Ims, UiT The Arctic University of Norway, Tromsø, Norway i Abstract Tundra arthropods have considerable ecological importance as a food source for several bird species that are reproducing in the Arctic. The actual arthropod taxa comprising the chick diet are however rarely known, complicating assessments of ecological interactions. In this study, I identified the nestling diet of Svalbard snow bunting (Plectrophenax nivalis) for the first time. Faecal samples of snow bunting chicks were collected in Adventdalen, Svalbard in the breeding season 2018 and analysed via DNA metabarcoding. Simultaneously, the availability of prey arthropods was measured via pitfall trapping. The occurrence of 32 identified prey taxa in the nestling diet changed according to varying abundances and emergence patterns within the tun- dra arthropod community: Snow buntings provisioned their offspring mainly with the most abundant prey items which were in the early season different Chironomidae (Diptera) taxa and Scathophaga furcata (Diptera: Scathophagidae), followed by Spilogona dorsata (Diptera: Mus- cidae). -

Survey to the Species of Family Sepsidae (Insecta: Diptera) in Iraq
International Journal of Science and Research (IJSR) ISSN (Online): 2319-7064 Index Copernicus Value (2015): 78.96 | Impact Factor (2015): 6.391 Survey to the species of Family Sepsidae (Insecta: Diptera) in Iraq Hanaa H. Al- Saffar Iraq Natural History Research Center and Museum, University of Baghdad, Baghdad, Iraq Abstract: The aim of this study is to survey species of Sepsidae family, The investigation showed three genera , date and locality of collecting specimens were recorded. Keywords: Acalybtarae, Black scavenger fly, Brachycera Diptera, Iraq, Sepsidae 1. Introduction 2. Materials and Methods The black scavenger flies is common name known on family The adult specimens were collected by sweeping net from Sepsidae (Diptera: Acalbtrata ) . The members of this family several region of Iraq , Baghdad, Najaf , Basra from field are worldwide distribution in all zoogeographical regions. near animal houses , and from carions of rabbit . After The family is represented about 339 species belonging to 38 collecting flies they killed by freezing for several hours , genera [1] then mounted with small label recorded the locality and date of collections and insect pins , they were keptq in insect box The sepsid flies are small –medium in size (2-12mm length). until diagnosis. For identification to genra and species using Most species are ant-like flies, with a narrow "waist[1] and taxonomic keys such as [2], [10], [21], [22]. The plates were morphologically and ecologically uniform family of the pictured by Dino Light microscope super family Sciomyzoidea [2],[3] 3. Results and Discussion The adults and larvae abundance in several dung of horses ,cows and other animals , and they associated with animal Family SEPSIDAE Walker, 1883 vertebrates carrion and human , decaying vegetations and other organic matter. -

Selection on Morphological Traits and Fluctuating Asymmetry by a Fungal Parasite in the Yellow Dung Fly
bioRxiv preprint doi: https://doi.org/10.1101/136325; this version posted September 14, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. 1 2 3 4 5 6 7 Blanckenhorn WU. 2017. 8 Selection on morphological traits and fluctuating asymmetry by a 9 fungal parasite in the yellow dung fly. 10 bioRxiv 136325, http://dx.doi.org/10.1101/136325 11 12 A Preprint reviewed and recommended by Peer Community 13 Evolutionary Biology: 14 http://dx.doi.org/10.24072/pci.evolbiol.100027 15 16 17 bioRxiv preprint doi: https://doi.org/10.1101/136325; this version posted September 14, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Wolf U. Blanckenhorn Selection by a fungal parasite in dung flies Selection on morphological traits and fluctuating asymmetry by a fungal parasite in the yellow dung fly WOLF U. BLANCKENHORN Department of Evolutionary Biology and Environmental Studies, University of Zürich, Winterthurerstrasse 190, CH-8057 Zürich, Switzerland; Fax: +41 44 635.4780; E-mail: [email protected] Abstract Evidence for selective disadvantages of large body size remains scarce in general. Previous phenomenological studies of the yellow dung fly Scathophaga stercoraria have demonstrated strong positive sexual and fecundity selection on male and female size. -

Master Thesis
Master thesis Effects of temperature, photoperiod and developmental stage on diapause in black scavenger flies (Diptera: Sepsidae) Valérian Zeender December 2015 Supervision by: Prof. Dr. Wolf U. Blanckenhorn University of Zurich Institute of Evolutionary Biology and Environmental Studies Valérian Zeender MSc Thesis University of Zurich Contents Abstract…………………………………………………………………………………………………………… 3 Résumé……………………………………………………………………………………………………….……4 1. Introduction……………………………………………………………………………………………………. 5 1.1 The adaptive significance of diapause 5 1.2 Environmental inducing cues 6 1.3 Reproductive diapause 7 1.4 Sepsid flies and research questions 8 2. Material and methods………………………………………………………………………………………...9 2.1 Choice of species 9 2.2 Rearing conditions 11 2.3 Experimental design 12 2.4 Female diapause assessment 14 2.5 Seasonal field sampling 15 2.5.1 Field-samples diapause assessment 16 2.6 Statistical analyses 16 3. Results…………………………………………………………………………………………………………18 3.1 Effects of temperature and photoperiod across species 18 3.2 Life stage effects within species 20 3.3 Seasonal changes in diapause incidence in S.cynipsea 23 4. Discussion…………………………………………………………………………………………………….25 4.1 Influence of thermal niche on diapause-induction mechanism 26 4.2 Life stage effect 27 4.3 Phylogenetic variation in diapause-induction mechanisms 28 4.4 Seasonal changes in diapause in S. cynipsea male and female………………..29 4.4.1 Males 29 4.4.2 Females 30 4.5 Molecular outlook 32 4.6 Conclusion 32 Acknowledgements…………………………………………………………………………………………….33 References……………………………………………………………………………………………………….34 Appendix……………………………………………………………………………………………………….. 39! Statement of authorship……………………………………………………………………………………..45! 2 Valérian Zeender MSc Thesis University of Zurich Abstract Temperature and photoperiod are the major environmental cues used by insects to determine the best timing to overwinter. However, the relative importance of each of these variables and the life stage at which individuals are sensitive to diapause induction cues remains largely unresolved. -

Repeatability of Sperm Size in Outbred and Inbred Scathophaga Stercoraria (Diptera: Scathophagidae)
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2007 Repeatability of sperm size in outbred and inbred Scathophaga stercoraria (Diptera: Scathophagidae) Bernasconi, G ; Ward, P I ; Hellriegel, B Abstract: Variability in male gametic traits can depend on several genetic and environmental factors such as developmental instability as a consequence of inbreeding, developmental noise during spermatogenesis, or age- or condition-dependent changes in allocation to sperm cells. Variation in sperm size is particularly evident in species that produce more than one sperm morph but also occurs among males in sperm- monomorphic species. Both discrete and continuous sperm size variation have been implicated in male fertilization success when the sperm of several males directly compete for fertilization of the same set of ova. In this study, we investigated among-male variation in sperm length in field-collected, outbred male Scathophaga stercoraria (L.) flies, as well as in flies from the same natural population that hadbeen subjected to 15 and 16 generations of inbreeding under laboratory conditions. Among-male variation in sperm length was significant and repeatable over subsequent matings in both inbred and outbred flies. We conclude that sperm length can be used as an individual male marker in sperm competition studies and that significant repeatability of sperm length supports heritability for this trait DOI: https://doi.org/10.4039/n06-063 Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-154393 Journal Article Published Version Originally published at: Bernasconi, G; Ward, P I; Hellriegel, B (2007). -

Behavioural Mechanisms of Reproductive Isolation Between Two Hybridizing Dung Fly Species
Animal Behaviour 132 (2017) 155e166 Contents lists available at ScienceDirect Animal Behaviour journal homepage: www.elsevier.com/locate/anbehav Behavioural mechanisms of reproductive isolation between two hybridizing dung fly species * Athene Giesen , Wolf U. Blanckenhorn, Martin A. Schafer€ Institute of Evolutionary Biology and Environmental Studies, University of Zurich, Zurich, Switzerland article info Characterization of the phenotypic differentiation and genetic basis of traits that can contribute to Article history: reproductive isolation is an important avenue to understand the mechanisms of speciation. We quan- Received 29 November 2016 tified the degree of prezygotic isolation and geographical variation in mating behaviour among four Initial acceptance 26 January 2017 populations of Sepsis neocynipsea that occur in allopatry, parapatry or sympatry with four populations of Final acceptance 21 June 2017 its sister species Sepsis cynipsea. To obtain insights into the quantitative genetic basis and the role of selection against hybrid phenotypes we also investigated mating behaviour of F1 hybrid offspring and MS. number: 16-01039R corresponding backcrosses with the parental populations. Our study documents successful hybridization under laboratory conditions, with low copulation frequencies in heterospecific pairings but higher fre- Keywords: quencies in pairings of F1 hybrids signifying hybrid vigour. Analyses of F1 offspring and their parental biogeography backcrosses provided little evidence for sexual selection against hybrids. -

A Fungal Parasite Selects Against Body Size but Not Fluctuating Asymmetry in Swiss Subalpine Yellow Dung Flies
Alpine Entomology 5 2021, 27–35 | DOI 10.3897/alpento.5.65653 A fungal parasite selects against body size but not fluctuating asymmetry in Swiss subalpine yellow dung flies Wolf U. Blanckenhorn1 1 Department of Evolutionary Biology and Environmental Studies, University of Zürich, Winterthurerstrasse 190, CH-8057, Zürich, Switzerland http://zoobank.org/0D01A23B-D327-4F92-9726-93AAD929CAFD Corresponding author: Wolf U. Blanckenhorn ([email protected]) Academic editor: Patrick Rohner ♦ Received 8 March 2021 ♦ Accepted 25 May 2021 ♦ Published 11 June 2021 Abstract Evidence for selective disadvantages of large body size remains scarce in general. Previous studies of the yellow dung fly Scatho- phaga stercoraria have demonstrated strong positive sexual and fecundity selection on male and female size. Nevertheless, the body size of flies from a Swiss study population has declined by ~10% 1993–2009. Given substantial heritability of body size, this neg- ative evolutionary response of an evidently positively selected trait suggests important selective factors being missed. An episodic epidemic outbreak of the fungus Entomophthora scatophagae permitted assessment of natural selection exerted by this fatal parasite. Fungal infection varied over the season from ~50% in the cooler and more humid spring and autumn to almost 0% in summer. The probability of dying from fungal infection increased with adult fly body size. Females never laid any eggs after infection, so there was no fungus effect on female fecundity beyond its impact on mortality. Large males showed their typical mating advantage in the field, but this positive sexual selection was nullified by fungal infection. Mean fluctuating asymmetry of paired appendages (legs, wings) did not affect the viability, fecundity or mating success of yellow dung flies in the field. -

Spineless Spineless Rachael Kemp and Jonathan E
Spineless Status and trends of the world’s invertebrates Edited by Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Spineless Spineless Status and trends of the world’s invertebrates of the world’s Status and trends Spineless Status and trends of the world’s invertebrates Edited by Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Disclaimer The designation of the geographic entities in this report, and the presentation of the material, do not imply the expressions of any opinion on the part of ZSL, IUCN or Wildscreen concerning the legal status of any country, territory, area, or its authorities, or concerning the delimitation of its frontiers or boundaries. Citation Collen B, Böhm M, Kemp R & Baillie JEM (2012) Spineless: status and trends of the world’s invertebrates. Zoological Society of London, United Kingdom ISBN 978-0-900881-68-8 Spineless: status and trends of the world’s invertebrates (paperback) 978-0-900881-70-1 Spineless: status and trends of the world’s invertebrates (online version) Editors Ben Collen, Monika Böhm, Rachael Kemp and Jonathan E. M. Baillie Zoological Society of London Founded in 1826, the Zoological Society of London (ZSL) is an international scientifi c, conservation and educational charity: our key role is the conservation of animals and their habitats. www.zsl.org International Union for Conservation of Nature International Union for Conservation of Nature (IUCN) helps the world fi nd pragmatic solutions to our most pressing environment and development challenges. www.iucn.org Wildscreen Wildscreen is a UK-based charity, whose mission is to use the power of wildlife imagery to inspire the global community to discover, value and protect the natural world. -
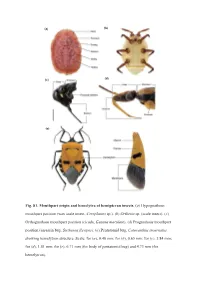
Page 1 (A) (E) Fig. S1. Mouthpart Origin and Hemelytra of Hemipteran
(a) (b) (d) (c) (e) Fig 61. Mouthpart origin and hemelytra of hemipteran insects. (a) Hypognathous mouthpart position (wax scale insect, Ceroplastes sp.). (b) Orthezia sp. (scale insect). (c) Orthognathous mouthpart position (cicada, Gaeana maculate). (d) Prognathous mouthpart position (assassin bug, Sirthenea flavipes). (e) Pentatomid bug, Catacanthus incarnatus showing hemelytron structure. Scale: for (a), 0.40 mm; for (b), 0.65 mm; for (c), 3.84 mm; for (d), 1.81 mm; for (e), 6.71 mm (for body of pentatomid bug) and 4.73 mm (for hemelytron). Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG1 PCG2 Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG3 RNA )LJ62. AliGROOVE analysis for codon positions of protein-coding genes (PCGs) and RNA genes. PCG1, the first codon position of PCGs. PCG2, the second codon position of PCGs. PCG3, the third codon position of PCGs. RNA, sequences of tRNA and rRNA genes. The mean similarity score between sequences is represented by a colored square, based on AliGROOVE scores from -1, indicating great difference in rates from the remainder of the data set, that is, heterogeneity (red coloring), to +1, indicating that ratesmatch all other comparisons (blue coloring). Bactericera sinica Sternorrhyncha Cicadomorpha Coleorrhyncha Fulgoromorpha Dipsocoromorpha Gerromorpha Enicocephalomorpha Nepomorpha Leptopodomorpha Cimicomorpha Heteroptera Pentatomomorpha Eusthenes cupreus FigS33K\ORJHQHWLFWUHHLQIHUUHGIURP3K\OR%D\HVDQDO\VLVRIWKH3&*51$GDWDVHWXQGHUWKe &$7*75PL[WXUHPRGHO9DOXHVDWQRGHVDUH%D\HVLDQ33V -

First Ever Report of a Bite by Nabis Argentinus Meyer-Dür (Hemiptera: Heteroptera: Nabidae) on a Human
Short Communication Biodiversity and Natural History (2017) Vol. 3, No. 2, 45-47 First ever report of a bite by Nabis argentinus Meyer-Dür (Hemiptera: Heteroptera: Nabidae) on a human Primer registro de una picadura de Nabis argentinus Meyer-Dür (Hemiptera: Heteroptera: Nabidae) en un ser humano Marcela Cornelis1, Fernando Diez1 & María del Carmen Coscarón2 1Universidad Nacional de La Pampa, Facultad de Ciencias Exactas y Naturales, Uruguay 151 L6300CLB, Santa Rosa, La Pampa, Argentina. 2Universidad Nacional de La Plata, Facultad de Ciencias Naturales y Museo, División Entomología, Paseo del Bosque s/n 1900, La Plata, Buenos Aires, Argentina. *Correspondence author: [email protected] Abstract Herein, we described the first ever reported bite of Nabis argentinus Meyer-Dür 1870 on a human. The bite was registered in the locality Santa Rosa La Pampa, Argentina (36°37'29.02"S, 64°17'19.13"W). The insect was not provoked by the victim, and thus, the bite was probably not in self-defense. We therefore concluded that the insect bite the victim because it was searching for sources of hydration. Key words: adventitious bite, nabids, predaceous habit, record, symptomatology. Resumen Se describe el primer caso de una picadura adventicia en un ser humano por Nabis argentinus Meyer-Dür 1870, en la localidad de Santa Rosa La Pampa, Argentina (36°37'29.02"S, 64°17'19.13"W). El insecto no fue provocado por la víctima, por lo que probablemente la picadura no fue en defensa propia. Por lo tanto, concluimos que el insecto picó a la víctima en búsqueda de recursos de hidratación. -

Age-Related Sperm Production, Transfer, and Storage in The
Age-related sperm production, transfer, and storage in the sweet potato weevil, cylas formicarius (fabricius) (coleoptera: Curculionidae) Authors: Satoshi Hiroyoshi, Tsuguo Kohama, and Gadi V. P. Reddy The final publication is available at Springer via http://dx.doi.org/10.1007/s10905-016-9590-0. Hiroyoshi, Satoshi, Tsuguo Kohama, and Gadi V P Reddy. "Age-related sperm production, transfer, and storage in the sweet potato weevil, cylas formicarius (fabricius) (coleoptera: Curculionidae)." Journal of Insect Behavior (November 2016). DOI:https://dx.doi.org/10.1007/ s10905-016-9590-0. Made available through Montana State University’s ScholarWorks scholarworks.montana.edu Age-Related Sperm Production, Transfer, and Storage in the Sweet Potato Weevil, Cylas formicarius (Fabricius) (Coleoptera: Curculionidae) Satoshi Hiroyoshi1,2 & Tsuguo Kohama 1,3 & Gadi V. P. Reddy4 1 Okinawa Prefectural Fruit-fly Eradication Program Office, 123 Maji, Naha, Okinawa 902-0072, Japan 2 7-12-203 Kotobukiso, Nishikawacho, Itoman, Okinawa 901-0304, Japan 3 Okinawa Prefectural Agricultural Research Center, 820 Makabe, Itoman, Okinawa 901-0336, Japan 4 Western Triangle Ag Research Center, Montana State University, 9546 Old Shelby Rd., P. O. Box 656, Conrad, MT 59425, USA Abstract The relationship between sperm production, insemination rate, and sperm transfer were studied in the sweet potato weevil, Cylas formicarius. Older adult males retained more sperm in the testes-seminal vesicle complex (TSC) and thus more was ejaculated into females at first mating. Number of matings per day for males was relatively constant across different ages, and frequent mating resulted in a reduced amount of sperm transferred to females, especially in young males.