Gentianaceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Summary of Offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019
Summary of offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019 3841 Number of items in BX 301 thru BX 463 1815 Number of unique text strings used as taxa 990 Taxa offered as bulbs 1056 Taxa offered as seeds 308 Number of genera This does not include the SXs. Top 20 Most Oft Listed: BULBS Times listed SEEDS Times listed Oxalis obtusa 53 Zephyranthes primulina 20 Oxalis flava 36 Rhodophiala bifida 14 Oxalis hirta 25 Habranthus tubispathus 13 Oxalis bowiei 22 Moraea villosa 13 Ferraria crispa 20 Veltheimia bracteata 13 Oxalis sp. 20 Clivia miniata 12 Oxalis purpurea 18 Zephyranthes drummondii 12 Lachenalia mutabilis 17 Zephyranthes reginae 11 Moraea sp. 17 Amaryllis belladonna 10 Amaryllis belladonna 14 Calochortus venustus 10 Oxalis luteola 14 Zephyranthes fosteri 10 Albuca sp. 13 Calochortus luteus 9 Moraea villosa 13 Crinum bulbispermum 9 Oxalis caprina 13 Habranthus robustus 9 Oxalis imbricata 12 Haemanthus albiflos 9 Oxalis namaquana 12 Nerine bowdenii 9 Oxalis engleriana 11 Cyclamen graecum 8 Oxalis melanosticta 'Ken Aslet'11 Fritillaria affinis 8 Moraea ciliata 10 Habranthus brachyandrus 8 Oxalis commutata 10 Zephyranthes 'Pink Beauty' 8 Summary of offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019 Most taxa specify to species level. 34 taxa were listed as Genus sp. for bulbs 23 taxa were listed as Genus sp. for seeds 141 taxa were listed with quoted 'Variety' Top 20 Most often listed Genera BULBS SEEDS Genus N items BXs Genus N items BXs Oxalis 450 64 Zephyranthes 202 35 Lachenalia 125 47 Calochortus 94 15 Moraea 99 31 Moraea -

AGCBC Seedlist2019booklet
! Alpine Garden Club of British Columbia Seed Exchange 2019 Alpine Garden Club of British Columbia Seed Exchange 2019 We are very grateful to all those members who have made our Seed Exchange possible through donating seeds. The number of donors was significantly down this year, which makes the people who do donate even more precious. We particularly want to thank the new members who donated seed in their first year with the Club. A big thank-you also to those living locally who volunteer so much time and effort to packaging and filling orders. READ THE FOLLOWING INSTRUCTIONS CAREFULLY BEFORE FILLING IN THE REQUEST FORM. PLEASE KEEP YOUR SEED LIST, packets will be marked by number only. Return the enclosed request form by mail or, if you have registered to do so, by the on-line form, as soon as possible, but no later than DECEMBER 8. Allocation: Donors may receive up to 60 packets and non-donors 30 packets, limit of one packet of each selection. Donors receive preference for seeds in short supply (USDA will permit no more than 50 packets for those living in the USA). List first choices by number only, in strict numerical order, from left to right on the order form. Enter a sufficient number of second choices in the spaces below, since we may not be able to provide all your first choices. Please print clearly. Please be aware that we have again listed wild collected seed (W) and garden seed (G) of the same species separately, which is more convenient for people ordering on-line. -

Chromosome Botany
“Chromosome Botany” is the organ journal of the International Society of Chromosome Botany (ISCB) and is a periodical devoted to the publication of original researches to study correlation between botanical function and phenomenon and chromosome and their nuclear DNA and furthermore total molecular genomic DNA by methodologies of molecular sequencing, molecular systematic, molecular cytogenetics, microscopic and electron microscopic observations as well as the traditional, orthodox aspects. The Society is established for the purposes of the progresses in researches and technologies, international exchanges and collaborations of personnels, enlightenment and education of our original knowledges in world-wide chromosome botany. <Titles of the articles of Chromosome Botany> Volume 12, Number 4 Apr. 2018 72-76. Chromosomal aberrations and Ac/Ds transposition in Garlic Rasha kamal Helmey and Gehan Mohamed Anwar < Garlic; Chromosomal aberration; Ac/Ds element > 77-85. Meiotic studies of the Convolvulaceae Juss. from Indian Hot Desert Ramanpreet and Raghbir Chand Gupta < Convolvulaceae, Chromosome counts, Meiosis, Rajasthan > 86-90. Gamma rays induced chromosomal aberrations in tomato (Solanum lycopersicum L.) Vishnu Prasad Bhala and Rakesh Chandra Verma < Tomato, Solanum lycopersicum L., Seed germination, Meiotic abnormalities, Gamma rays > 91-94. Effect of colchicine on meiosis of Chrysanthemum carinatum Schousb. (Asteraceae) Rakesh Chandra Verma, Rakesh Purbiya and Rekha Solanki < Colchicine, Chrysanthemum carinatum, Meiosis, Translocation, APG-IV > Volume 12, Number 3 Nov. 2017 41-45. Cytogenetic investigations in colchicine induced tetraploid of Cosmos sulphureus (Asteraceae) Rakesh Chandra Verma, Preeti Dass, Nilofar Shaikh and Mushtaq Ahmad Khah < Cosmos sulphureus, colchiploid, quadrivalent, capitulum > 46-51. Genomic distortion induced by food dyes on meristematic cells of Trachyspermum ammi (L.) Sprague (Ajwain) Harshita Dwivedi and Girjesh Kumar < Tatrazine, Sunset yellow, Trachyspermum ammi, Genotoxicity, Chromosomal anomalies> 52-55. -

Wonderful Plants Index of Names
Wonderful Plants Jan Scholten Index of names Wonderful Plants, Index of names; Jan Scholten; © 2013, J. C. Scholten, Utrecht page 1 A’bbass 663.25.07 Adansonia baobab 655.34.10 Aki 655.44.12 Ambrosia artemisiifolia 666.44.15 Aalkruid 665.55.01 Adansonia digitata 655.34.10 Akker winde 665.76.06 Ambrosie a feuilles d’artemis 666.44.15 Aambeinwortel 665.54.12 Adder’s tongue 433.71.16 Akkerwortel 631.11.01 America swamp sassafras 622.44.10 Aardappel 665.72.02 Adder’s-tongue 633.64.14 Alarconia helenioides 666.44.07 American aloe 633.55.09 Aardbei 644.61.16 Adenandra uniflora 655.41.02 Albizia julibrissin 644.53.08 American ash 665.46.12 Aardpeer 666.44.11 Adenium obesum 665.26.06 Albuca setosa 633.53.13 American aspen 644.35.10 Aardveil 665.55.05 Adiantum capillus-veneris 444.50.13 Alcea rosea 655.33.09 American century 665.23.13 Aarons rod 665.54.04 Adimbu 665.76.16 Alchemilla arvensis 644.61.07 American false pennyroyal 665.55.20 Abécédaire 633.55.09 Adlumia fungosa 642.15.13 Alchemilla vulgaris 644.61.07 American ginseng 666.55.11 Abelia longifolia 666.62.07 Adonis aestivalis 642.13.16 Alchornea cordifolia 644.34.14 American greek valerian 664.23.13 Abelmoschus 655.33.01 Adonis vernalis 642.13.16 Alecterolophus major 665.57.06 American hedge mustard 663.53.13 Abelmoschus esculentus 655.33.01 Adoxa moschatellina 666.61.06 Alehoof 665.55.05 American hop-hornbeam 644.41.05 Abelmoschus moschatus 655.33.01 Adoxaceae 666.61 Aleppo scammony 665.76.04 American ivy 643.16.05 Abies balsamea 555.14.11 Adulsa 665.62.04 Aletris farinosa 633.26.14 American -
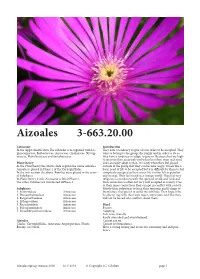
Aizoales 3-663.20.00
Aizoales 3-663.20.00 Taxonomy Introduction In the Apg2 classifcation Te suborder is recognised with Lo- Tey have a tendency to give a lot in order to be accepted. Tey phiocarpaceae, Barbeuiaceae, Aizoaceae, Gisekiaceae, Nyctag- want to belong to the group, the family and in order to do so inaceae, Phytolaccaceae and Sarcobataceae. they have a tendency to adapt, to give in. Because they are high- ly sensitive they accurately feel what the others want and need Plant theory and can easily adapt to that. It is only when they feel placed In the Plant theory the above clade is given the name Aizoales. outside of the group that they can become angry. It feels like a Aizoales is placed in Phase 2 of the Caryophyllidae. basic need of life to be accepted but it is difcult for them to feel In the frst version the above Families were placed in the sever- completely accepted as their inner life is ofen felt as peculiar al Subphases. and strange. Tey feel weird in a strange world. Tey feel very In Plant theory 2 only Aizoaceae is lef inPhase 2. religious, a connection with the spiritual world and God and Te other Families are transferred toPhase 3. that connection is ofen not very well accepted in society. Due to their inner convictions they can get in confict with society. Subphases Mostly their solution is to keep their opinions and feelings to 1. Sesuvioideae Aizoaceae themselves; they prefer to avoid the conficts. Tey hope to be 2. Drosanthemoideae Aizoaceae be able to stay with their own inner convictions and that they 3. -

2019–2020 Seed Exchange Catalog 1 Agastache – Allium
MID-ATLANTIC GROUP 2019 –2020 Seed Exchange Catalog The 26th annual edition of the Seed Exchange Our Seed Donors Catalog includes 868 seed donations contrib- Catalog listed seed was generously contributed by our members. Where the initial source name is followed by “/”and other member uted by 54 gardeners, from beginners to pro- names, the latter identifies those who actually selected, collected, fessionals. Over 126 new plants were donated cleaned, and then provided descriptions to the members who pre- pared the catalog. If a donor reported their zone, you will find it in for the first time. As you can see, this seed parenthesis. Our sincere thanks to our donors—they make this Seed program includes new plants not previously Exchange possible. offered as well as old favorites. Axel, Laura 3132 Maher, Carole 3176 (7a) Bartlett, John 45 Mahony, Peter 590 (7a) We’re sure you’ll enjoy perusing this year’s Bartram’s Garden 9975 Malarek, David 2608 selections and you will find plants your gar- Berger, Clara 65 Malocsay, Jan-Paul 592 (6) Biderman/Barron 43 Marzocco, Sharon 1066 den can’t do without! Since some listed seed Bittmann, Frank 2937 (6a) Matlack, Nancy 3616 is in short supply, you are encouraged to Bowditch, Margaret 84 McGowan, Brian 3666 Boylan, Rebecca 2137 McShane, Nadeen 627 place your order early. Bricker, Matthew 2429 Nachlas, Sally 2621 Carey, Jenny Rose 1918 Norfolk Botanical staff 1999 (8a) Chapman, Marcia Perron, William 3321 (6) Our Catalog Staff Cresson, Charles 199 (7) Plant Delights 32 The HPS members who have worked to produce this catalog, Creveling, Beth 200 (7) Roper, Lisa 9968 over the last three months, form a talented and dedicated DeMarco, Loretta 215 (7b) Scofield, Connie 1585 group to whom we are all grateful. -
Jan Scholten Wonderful Plants Reading Excerpt Wonderful Plants of Jan Scholten Publisher: Alonnissos Verlag
Jan Scholten Wonderful Plants Reading excerpt Wonderful Plants of Jan Scholten Publisher: Alonnissos Verlag http://www.narayana-verlag.com/b14446 In the Narayana webshop you can find all english books on homeopathy, alternative medicine and a healthy life. Copying excerpts is not permitted. Narayana Verlag GmbH, Blumenplatz 2, D-79400 Kandern, Germany Tel. +49 7626 9749 700 Email [email protected] http://www.narayana-verlag.com Table of Contents 0.9.06 Stage-6 40 0.1.1 Publication data 3 0.9.07 Stage-7 40 0.1.2 Table of Contents 13 0.9.08 Stage-8 40 0.1.3 Word of thanks 14 0.9.09 Stage-9 41 0.1.4 Foreword Klein 14 0.9.10 Stage-10 41 0.1.5 Foreword Kuiper 15 0.9.11 Stage-11 41 0.1.6 Introduction 16 0.9.12 Stage-12 42 0.1.7 Introduction use 16 0.9.13 Stage-13 42 0.1.8 Use 17 0.9.14 Stage-14 42 0.2 Goal 18 0.9.15 Stage-15 43 0.3.1 Method 19 0.9.16 Stage-16 43 0.3.2 Element Theory 19 0.9.17 Stage-17 43 0.3.3 Classification of Plants 20 0.9.18 Stage-18 44 0.3.4 Classes 20 000.00 Evolution 44 0.4 Result 21 000.00.00 Kingdom 45 0.4.0 Result 21 000.00.00 Plant Kingdom 47 0.4.1 Phyla and Series 21 000.00.20 Kingdom 49 0.4.2 Classes and Series 22 111.00.00 Archaeoplastidae 51 0.4.3 Subclasses and Series 22 111.02.20 Fucus vesiculosus 51 0.4.4 Orders and Phases 23 111.10.00 Rhodophyta 51 0.4.5 Families and Subphases 23 111.10.13 Helminthochortos 51 0.4.7 Number 23 111.10.20 Chondrus crispus 51 0.5 Discussion 24 111.10.20 Porphyra yezoensis 51 0.5.0 Discussion 24 112.20.00 Glaucophyta 51 0.5.1 Discussion Apg 3 24 210.00.00 Chlorophyta 51 0.5.1 -
Forgotten Carpological Collection of Professor Ivo Horvat Discovered and Digitized
NAT. CROAT. VOL. 30 No 1 269–287 ZAGREB July 31, 2021 professional paper / stručni rad DOI 10.20302/NC.2021.30.19 FORGOTTEN CARPOLOGICAL COLLECTION OF PROFESSOR IVO HORVAT DISCOVERED AND DIGITIZED SARA EsseRT1, VEDRAN ŠEGOTA2*, IVANA GOD3, NORA MAS4, 5 5 Daniel Špoljarić & Maja popović 1* 1Division of Botany, Department of Biology, Faculty of Science, University of Zagreb, Marulićev trg 20/II, HR-10000 Zagreb, Croatia 2ZA & ZAHO herbarium collections, Division of Botany, Department of Biology, Faculty of Science, University of Zagreb, Marulićev trg 20/II, HR-10000 Zagreb, Croatia 3Ivan Kukuljević Sakcinski Elementary School, Akademika Ladislava Šabana 17, HR-42240 Ivanec, Croatia 4Department of Animal Nutrition and Dietetics, Faculty of Veterinary Medicine, University of Zagreb, Heinzelova 55, HR-10000 Zagreb, Croatia 5Department of Veterinary Biology, Faculty of Veterinary Medicine, University of Zagreb, Heinzelova 55, HR-10000 Zagreb, Croatia Essert, S., Šegota, V., God, I., Mas, N., Špoljarić, D. & Popović, M.: Forgotten carpological collection of Professor Ivo Horvat discovered and digitized. Nat. Croat. Vol. 30, No. 1., 269–287, 2021, Zagreb. The carpological collection of Professor Ivo Horvat, a famous Croatian botanist of 20th century, was saved from oblivion, after its unexpected discovery at the Faculty of Veterinary Medicine in Zagreb in 2019. As many as 515 diaspores (fruits and seeds) of 486 vascular plant taxa were systema- tised and digitised and the nomenclature was updated. A comprehensive comparison of Horvat’s carpological and herbarium collection (ZAHO) revealed a large amount of overlap. A large photo-cat- alogue was created and will be publicly accessible through the Flora Croatica Database. -

Alpine Garden Club of British Columbia Seed Exchange 2020
Alpine Garden Club of British Columbia Seed Exchange 2020 Alpine Garden Club of British Columbia Seed Exchange 2020 We are very grateful to all those members who have made our Seed Exchange possible through donating seeds, and to those living locally who volunteer so much time and effort to packaging and filling orders. Read the following instructions carefully before filling out the seed request form. PLEASE KEEP YOUR SEED LIST, packets will be marked by number only. Please send the Seed Exchange Request Form (last page of booklet) by mail as soon as possible, but postmarked no later than DECEMBER 6. We prefer that you request your seeds on-line (see back page) and the complete seed list is available on the website for reference. Although the deadline is in December, we would very much appreciate it if people could order early whenever possible. With the restrictions due to the COVID-19 pandemic, the whole distribution process has to be changed, and knowing how many packets of each species are required will help. Allocation: Members are entitled to 30 packets of seeds and qualified donors can request up to 60 packets. Given the challenges of seed packaging this year by our team of volunteers, please consider that it is not necessary to request your full complement of seeds. Donors receive preference for seeds in short supply. (USDA will permit no more than 50 packets for those living in the USA.) To qualify as a donor, a minimum donation of five different species is required. North American members should donate this minimum in seeds native to North and South America. -

Spring 2014 Mail Order Catalog Cistus Nursery
Spring 2014 Mail Order Catalog Cistus Nursery 22711 NW Gillihan Road Sauvie Island, OR 97231 503.621.2233 phone order by phone 9 - 5 pst, visit 10am - 5pm, mail, or email: [email protected] 24-7-365 www.cistus.com Spring 2014 Mail Order Catalog 2 Abelia x grandiflora 'Margarita' margarita abelia New and interesting abelia with red stems bearing variegated leaves, green with bright yellow margins, dressing up a smallish, evergreen shrub, to about 4 ft tall and wide. A cheerful addition to the garden as a single specimen or low-growing hedge. Flowers are white, typical of the species, and begin in May, continuing sporadically throughout the season. Best in sun -- they tend to be leggy in shade -- with average summer water. Frost hardy to -10F, USDA zone 6. $14 Caprifoliaceae Abutilon 'Savitzii' flowering maple One of the few abutilons we sell that is quite tender. Grown since the 1800s for its wild variegation -- the leaves large and pale, almost white with occasional green blotches -- and long, salmon-orange, peduncled flowers. A medium grower, to 4-6 ft tall, needing consistent water and nutrients in sun to part shade. Winter mulch increases winter toughness as does some overstory. Frost hardy to 25 F, mid USDA zone 9. Where temperatures drop lower, best in a container or as cuttings to overwinter. Well worth the trouble! $9 Malvaceae Abutilon megapotamicum 'Ines' flowering maple The pale yellow, nearly white flowers of 'Ines' -- flared and backed by a dark red calyx -- are striking and abundant from spring through first frost. This new flowering maple is a fast-growing, medium shrub, to 5 ft tall x 5 ft wide, with slightly fuzzy leaves. -
SANPC Additions and Updates April2019
South African National Plant Checklist Report on additions and updates made to the South African National Plant Checklist during the period 4/2019–12/2019 Contents Names added ..............................................................................................................................2 Taxonomic status change: accepted names that became synonyms ..................................... 51 Taxonomic status change: synonyms that became accepted names ..................................... 66 Publications incorporated ....................................................................................................... 71 Report compiled on 7/1/2020 by Dr Ronell R Klopper SA National Plant Checklist Co-ordinator and Mr Pieter J.D. Winter Deputy SA National Plant Checklist Co-ordinator Alien Plant Checklist Co-ordinator Biosystematics & Biodiversity Collections Division South African National Biodiversity Institute 1 Names added * = naturalised alien taxa ACANTHACEAE ACANTHOPSIS HARV. Acanthopsis pagodiformis H.M.Steyn, Fl. Pl. Africa 66: 145 (2019); Type: South Africa, Northern Cape, Namaqualand District, Richtersveld, Rooiberg NE of Eksteenfontein, Steyn 2147 (PRE, holo.; KMG, NBG, iso.) AGAVACEAE ANTHERICUM L. Anthericum exuviatum Jacq., Icon. Pl. Rar. 2 (13): 18, t. 415 (1794); = Drimia exuviata (Jacq.) Jessop, J. S. African Bot. 43(4): 276 (1977), Type: illustration in Jacq., Icon. Pl. Rar. 2: t. 415 (1794). Anthericum filifolium Thunb., Prodr. Pl. Cap. 1: 62 (1794), nom.illegit., non Jacq. (1794); = Ornithogalum thunbergii Kunth, Enum. Pl. [Kunth] 4: 369 (1843), nom.nov. pro Anthericum filifolium Thunb. (Oct 1794) et Anthericum nematodes Schult. & Schult.f. (1829) Anthericum filifolium Jacq., Icon. Pl. Rar. 2 (15): 18, t. 414 (1794), non Thunb. (Oct 1794); Type: illustration in Jacq., Icon. Pl. Rar. 2 (15): t. 414 (1794). = Drimia exuviata (Jacq.) Jessop, J. S. African Bot. 43(4): 276 (1977), Type: illustration in Jacq., Icon. Pl. Rar. 2: t. 415 (1794). -
Galtonia Candicans – Новая Культура На Юге Западной Сибири
Глава 2. Интродукция и сортоизучение Kazakhmedov R. E., Kafarova N. M. “Dagestan Breeding Experimental Station of Viticulture and Vegetable Growing” Branch of the Federal State Budgetary Scientific Institution “North Caucasian Federal Research Centre for Horticulture, Viticulture, Wine-making”, c. Derbent, Russia, e-mail: [email protected] the paper presents a composition and range of subtropical fruit crops from the collection of Dagestan Breeding experimental Station of Viticulture and Vegetable Growing. For the first time in the conditions of Southern Dagestan, based on the collection, there was given agrobiological and economic-technological evaluation for different species and cultivars of subtropical fruit crops (kaki persimmon, Oriental persimmon, american persimmon, pomegranate, fig, jujube, sea buckthorn, feijoa, cudrang, buffalo berry), which is of great interest for their implementation and cost- effective industrial cultivation in Dagestan. Some cultivars with high and regular yield, good quality and keeping quality of production were revealed. the paper describes the best selected cultivars of kaki persimmon, pomegranate, fig, jujube, sea buckthorn, feijoa, cudrang and buffalo berry that meet the requirements of production. Key words: collection, subtropical fruit and berry crops, cultivar, introduction, re- sistance to diseases and pests. УДК 582.572.227+581.543 GALTONIA CANDICANS – новая культура НА югЕ Западной СИБИРИ Клементьева л. А. Федеральное государственное бюджетное научное учреждение «Федеральный Алтайский научный центр агробиотехнологий», г. Барнаул, Россия, e-mail: [email protected] Приведены особенности сезонного развития Galtonia candicans (Baker) Decne. при первичной интродукции в условиях лесостепной зоны юга За- падной Сибири. луковичный многолетник родом из субтропической зоны южной Африки, не зимующий в грунте, проявил нетребовательность к пло- дородию почвы, ежегодно цвёл и плодоносил.