Thesis Chapter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Study of Water Quality of Swarnrekha River, Ranchi, Jharkhand, India
AEGAEUM JOURNAL ISSN NO: 0776-3808 Study of water quality of Swarnrekha River, Ranchi, Jharkhand, India Suresh Kumar 1, Sujata kumari 2 1. Dr. Shyama Prasad Mukherjee University, Ranchi, Jharkhand, India 2. Ranchi University, Ranchi, Jharkhand, India Corresponding Author: [email protected] Abstract The name of river “Swarnrekha” is given after in the ancient period due to the occurrence of “gold streaks” in the river water or river sediments. The river originated from a “seepage cum underground well”, locally called “Ranichuan” at the Nagari village of the Ranchi district. It is the first river which originates from seepage well locally called “Chuan” basically a great seepage well having a catchment area. According to Hindu Mythology, it is said that this Ranichuan was carved by the lord Rama by his arrow while Sita, his wife, was feeling thirsty during the period of Ramayana. In this way, we say that the river is basically originated from the seepage water or ground water. It travels towards the south east of Ranchi to East Singhbhum to Sarikhela and finally confluence with Damodar River at cretina mouth of the river. Previously, it was very pure form of drinkable water and day by day its quality deteriorated due to the anthropogenic activities and ultimately whole stretches of the river turned into garbage field and most polluted water streams. So, now we can say the river turned from gold streak to garbage streak and not suitable for the human beings without treatment. Physical, Chemical and microbial properties of the river water from point of origin to the lower chutia is deterioted such as water is very clean at the site of origin and became gradually hazy and dirt as it crosses through the habitants or settlements. -

CHALLENGES in EFFICIENT WATER MANAGEMENT in DAMODAR RIVER VALLEY - ROLE of DVC 1 2 Dipankar Chaudhuri ; Satyabrata Banerjee
CHALLENGES IN EFFICIENT WATER MANAGEMENT IN DAMODAR RIVER VALLEY - ROLE OF DVC 1 2 Dipankar Chaudhuri ; Satyabrata Banerjee Abstract The Damodar River Valley has an extensive history of developmental and planning activities since 1863. DVC was formed in the year 1948 by the act of Parliament to carry out the responsibilities for monitoring and developing this large watershed in an integrated manner. It is well aware that all the projects, planned originally could not be implemented till date by the DVC and the participatory states. Again, silt depositions in the existing reservoirs and channels due to erosions at the upper valley have reduced their respective storage and flowing capacities remarkably. On the other hand, demand of water has been increased many folds within the valley due to growth of industries, population etc. With passing of time, socio- economic and political condition of the valley has also changed a lot. So, considering the different constraints, DVC is trying to manage all its statutory obligations with its limitations. Performances of the operations in the different fields of activities like flood moderation, irrigation, municipal and irrigation water supply, Hydropower etc. have been studied elaborately in this article. Lots of new commendable initiatives to restore the lost-capacities and to increase the storage facilities have also been discussed. A Master Plan of the ecofriendly sustainable developmental activities of the valley in the different projected scenarios has already been prepared by DVC which has also been described in brief. Some scopes have been identified to take up a few new small Hydro schemes at different locations in the upper valley. -

River Action Plan Damodar
ACTION PLAN FOR REJUVENATION OF DAMODAR RIVER IN JHARKHAND JHARKHAND STATE POLLUTION CONTROL BOARD, DHURWA, RANCHI, JHARKHAND-834004 CONTENT CHAPTER I ❖ BACKGROUND ❖ INTRODUCTION ❖ PHYSIOGRAPHY ❖ WATER RESOURCES & RAINFALL ❖ ANNUAL RAINFALL ❖ DEVIATION OF RAINFALL ❖ SEASONAL RAINFALL ❖ RAINFALL TREND IN RABI SEASON ❖ AVERAGE MOTHLY RAINFALL ❖ MOVING AVERAGE OF THE RAINFALL ❖ EXTREME EVENT ANALYSIS ❖ SURFACE WATER RESOURCES ❖ GROUND WATER RESOURCES ❖ DRAINAGE SYSTEM AND MAPS CHAPTER II DAMODAR RIVER BASIN RIVER COURSE AND MAJOR TRIBUTARIES CHAPTER III- SOCIO-ECONOMIC IMPORTANCE ❖ WATER RESOURCES AND ITS USES ❖ MINING AND INDUSTRIAL ACTIVITIES ❖ NATURAL AND ANTHROPOGENIC HAZARDS ❖ IDENTIFIED STRETCHES FOR REDUCING POLLUTION CHAPTER IV- ACTION PLAN ❖ ACTION PLAN- SHORT TERM AND LONG TERM ACTION AND THE IDENTIFIED AUTHORITIES FOR INITIATING ACTIONS AND THE TIME LIMITS FOR ENSURING COMPLIANCE ❖ SHORT TERM AND LONG TERM ACTION PLANS FOR REJUVENATION OF RIVERS AND THE IMPLEMENTING AGENCIES RESPONSIBLE FOR EXECUTION OF THE ACTION PLANS AND THE TIME LIMITS ARE GIVEN IN TABLE AS BELOW ❖ PROPOSED ACTION PLAN BY VARIOUS DEPARTMENT OF GOVT. OF JHARKHAND ❖ PROPOSED ACTION PLAN FOR RESTORATION OF JHARKHAND RIVERS ❖ ACTION PLAN AT VILLAGE LEVEL ❖ TIMELINES FOR IMPLEMENTATION OF MODEL RESTORATION PLAN in 2019- 2020 and 2020-2021 Chapter-1 JHARKHAND & ITS WATER RESOURCES 1.1 BACKGROUND:-Hon’ble National Green Tribunal passed the following orders in OA No. 673/2018 & M.A. No. 1777/2018 titled News item published in “The Hindu “authored by Shri Jacob Koshy titled “More river stretches are now critically polluted: CPCB on 20.09.2018 as per excerpts below. “The issue taken up for consideration in this matter is abatement of pollution in 351 river stretches in the country, identified as such by the Central Pollution Control Board (CPCB). -

Damodar : a River Valley of Sorrow in Jharkhand State of India The
Damodar : A River Valley of Sorrow in Jharkhand state of India The Damodar is an inter-state river in the state of Jharkhand in India. In general rivers are feminine but Damodar is an exception. Like other two rivers -The Sonebhadra and The Brahmaputra Damodar is also categorised as a Masculine River. It emerges from the roots of an old tree, know as Pakar tree in local dialect, of extremists infested Boda Hills at "Kuru" Block of "Lohardaga " District in Jharkhand and merges into River "Bhagirathi " after traversing a total length of 541 Kms of which 258 Kms lies in the Jharkhand and the rest in West Bengal province. The total catchments area of the Damodar river system is 22,528 Sq Kms of which 16,934 Sq Kms (76 percent) is in the state of Jharkhand. The average yield of the Damodar River basin is 12.20 and its total surface flow in Jharkhand is estimated to be 5.80 Lham at 75 percent dependability as reported by the Irrigation Commission, Govt. of India 1972. Tributaries :- Its important tributaries are Barakar, Konar Bokaro and Gowai. The "barakar river " is its main tributary running almost parallel to it and joins it at 258 Kms near panchet at the border of Jharkhand and west Bengal where as its another left bank tributary "river Konar " merges into it at 180 kms from its origin near Bermo in Bokaro district of Jharkhand. The Bokaro and Konar rivers rise very near to each other on the Hazaribagh plateau and the two together meet meet before they finally outfall into Damodar at above 5 Kms further downstream. -

The World Bank
Public Disclosure Authorized Public Disclosure Authorized The World Bank ' Public Disclosure Authorized INTERNATIONAL BANK FOR RECONSTRUCTION AND DEVELOPMENT Washington, D. C. October, 1960 Public Disclosure Authorized THE WORLD BANK IN ASIA A Summary of Activities OCTOBER, 1960 THE WORLD BANK IN ASIA A Summary of Activities Table of Contents Pages Introduction 1 - 6 Burma 7 - 9 Ceylon 10 - 13 India 14 - 26 Japan 27 - 39 Malaya 40 - 42 Pakistan 43 - so Philippines 51 - 52 Thailand 53 - 58 The Indus Water Tr eaty, 1960 59 - 72 THE WORLD BANK IN ASIA Introduction In t his booklet Asia has been arbitrarily defined as extending from Japan and the Philippines t o the western border of Pakistan. The r egion incl udes t hirteen member countries of the Bank, which has been active in all of them, and has made loans in ei ght. The total lent up to t he end of September 1960, net of cancellations or r efundings , was made up as follows: Amounts net of Country Cancellations or r efundings Burma 19,350, 000 Ceyl on 23 ,900 ,000 India 662,100,000 Japan 337,400,000 Malaya 35,6oo,ooo Pakistan 241,300 ,000 Philippines 18,500, 000 Thailand 106, 650, 000 ~1,444,800,000 This lending, amounting to well over a quarter of t otal Bank loans in all member countries, has been concentrated on the development of basic services . For exampl e , loans for transporta t ion by r oad, r ai l , sea and air amount to near l y two- fifths of the total . -

The Lower Damodar River, India Advances in Asian Human-Environmental Research
The Lower Damodar River, India Advances in Asian Human-Environmental Research Series Editor Prof. Marcus Nüsser South Asia Institute, University of Heidelberg, Germany Editorial Board Prof. Eckart Ehlers, University of Bonn, Germany Prof. Harjit Singh, Jawaharlal Nehru University, New Delhi, India Prof. Hermann Kreutzmann, Freie Universität Berlin, Germany Prof. Ken Hewitt, Waterloo University, Canada Prof. Urs Wiesmann, University of Bern, Switzerland Prof. Sarah J. Halvorson, University of Montana, USA Dr. Daanish Mustafa, King’s College London, UK Aims and Scope The series aims at fostering the discussion on the complex relationships between physical landscapes, natural resources, and their modification by human land use in various environments of Asia. It is widely acknowledged that human-environment- interactions become increasingly important in area studies and development research, taking into account regional differences as well as bio-physical, socioe- conomic and cultural particularities. The book series seeks to explore theoretic and conceptual reflection on dynamic human-environment systems applying advanced methodology and innovative research perspectives. The main themes of the series cover urban and rural landscapes in Asia. Examples include topics such as land and forest degradation, glaciers in Asia, mountain environments, dams in Asia, medical geography, vulnerability and mitigation strategies, natural hazards and risk management concepts, environmental change, impacts studies and consequences for local communities. The relevant themes of the series are mainly focused on geographical research perspectives of area studies, however there is scope for interdisciplinary contributions. For further volumes: http://www.springer.com/series/8560 The Lower Damodar River, India Understanding the Human Role in Changing Fluvial Environment by Kumkum Bhattacharyya University of Michigan, Ann Arbor, MI, USA 123 Dr. -

A Less Resourced Language Spoken in the State of Jharkhand, India
Dialectologia 25 (2020), 25-43. ISSN: 2013-2247 Received 21 September 2018. Accepted 21 November 2018. DESIGNING A LINGUISTIC PROFILE OF KHORTHA: A LESS RESOURCED LANGUAGE SPOKEN IN THE STATE OF JHARKHAND, INDIA Atul AMAN1, Niladri Sekhar DASH1 & Jayashree CHAKRABORTY2 Linguistic Research Unit, Indian Statistical Institute, Kolkata1** Dept. of HSS, Indian Institute of Technology, Kharagpur2 ** [email protected] / [email protected] / [email protected] Abstract This paper describes the linguistic outline of Khortha language, which is spoken in the state of Jharkhand, India. Khortha is the second most spoken language after Hindi in the state of Jharkhand, with approximately 80 million speakers (As per the Govt. of India, census reports 2011). The paucity of the language resources in Khortha played a vital role in motivating us for the present work. The methodology adopted for the present study comprises linguistic field surveys (Dash & Aman 2015) and reviews on the earlier literature of Khortha. The current status and demographic profile of Khortha suggest its usage as a link language among the other indigenous language communities (i.e. Munda, Bedia, Kurmali, etc.) as well. The scope (usage) of the Khortha language within the various domains (i.e. administration, education, mass media, social divisions and religion, judiciary and interpersonal communication), as discussed in the paper, gives a clear idea of its usage and linguistic identity. This paper can be a helpful resource for the researchers in order to portray the current linguistic status of the language. Keywords Khortha, language resources, earlier literature, demographic profile, linguistic identity ** Linguistic Research Unit, Indian Statistical Institute, 203, B.T Road, Kolkata, 700108, West Bengal, India. -
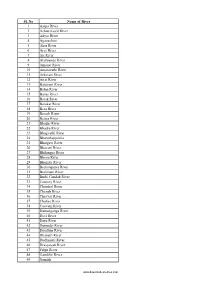
List of Rivers in India
Sl. No Name of River 1 Aarpa River 2 Achan Kovil River 3 Adyar River 4 Aganashini 5 Ahar River 6 Ajay River 7 Aji River 8 Alaknanda River 9 Amanat River 10 Amaravathi River 11 Arkavati River 12 Atrai River 13 Baitarani River 14 Balan River 15 Banas River 16 Barak River 17 Barakar River 18 Beas River 19 Berach River 20 Betwa River 21 Bhadar River 22 Bhadra River 23 Bhagirathi River 24 Bharathappuzha 25 Bhargavi River 26 Bhavani River 27 Bhilangna River 28 Bhima River 29 Bhugdoi River 30 Brahmaputra River 31 Brahmani River 32 Burhi Gandak River 33 Cauvery River 34 Chambal River 35 Chenab River 36 Cheyyar River 37 Chaliya River 38 Coovum River 39 Damanganga River 40 Devi River 41 Daya River 42 Damodar River 43 Doodhna River 44 Dhansiri River 45 Dudhimati River 46 Dravyavati River 47 Falgu River 48 Gambhir River 49 Gandak www.downloadexcelfiles.com 50 Ganges River 51 Ganges River 52 Gayathripuzha 53 Ghaggar River 54 Ghaghara River 55 Ghataprabha 56 Girija River 57 Girna River 58 Godavari River 59 Gomti River 60 Gunjavni River 61 Halali River 62 Hoogli River 63 Hindon River 64 gursuti river 65 IB River 66 Indus River 67 Indravati River 68 Indrayani River 69 Jaldhaka 70 Jhelum River 71 Jayamangali River 72 Jambhira River 73 Kabini River 74 Kadalundi River 75 Kaagini River 76 Kali River- Gujarat 77 Kali River- Karnataka 78 Kali River- Uttarakhand 79 Kali River- Uttar Pradesh 80 Kali Sindh River 81 Kaliasote River 82 Karmanasha 83 Karban River 84 Kallada River 85 Kallayi River 86 Kalpathipuzha 87 Kameng River 88 Kanhan River 89 Kamla River 90 -

Need for Integrated River Basin Management in the Context of West Bengal Floodplains
Journal of Management & Public Policy, Vol. 2, No. 1 December 2010 Need for Integrated River Basin Management in the Context of West Bengal Floodplains Sujit Choudhury *, Girija Sankar Chattopadhyay ** & Deepankar Chakrabarti *** Abstract Integrated river basin management is a very important issue across the globe for water conservation and sustainable development. The rivers in India are very critical for the development of the country for meeting the water requirement and maintaining the ecological balance. West Bengal, a state having large numbers of rivers, is facing problems associated with the vast floodplains of these rivers. During the last fifty years various riparian systems and their parts in West Bengal are getting affected by different developmental activities. The river floodplains are the worst effected parts in West Bengal with rapid changes of land use characters of younger and older floodplain areas. For meeting the water demand and flood control purpose number of structural measures like large dams and reservoirs are built on these rivers. This has reduced the periodic floods in downstream areas and provides irrigation in the fertile floodplain areas. But these are leading to the rapid changes of the land use characters of the floodplains of West Bengal. These measures and water withdrawal in different rivers of Bengal results in reduction of flow amplitude, increase in base flow variation, alteration in temperature regime and decline of mass transport of materials. With this the overall connectivity between upstream and downstream reaches are compromised. This has a detrimental effect on biodiversity and ecological processes. Sophisticated studies of the dynamic interrelationships among topographic, hydraulic, hydrologic, agricultural, and economic factors are necessary for immediate impact assessment. -

Training of Fulta James and Mary Reach of Hooghly
TRAINING OF FULTA JAMES AND MARY REACH OF HOOGHLY by M G HiYanandani and S T Ghotankar Technical Memorandum NAV 1 September 1961- GOVERNMEN'l' OF INDIA CENTRAL WATER ANP POWER RESEARCH STATION Poona, India Fulta James and Mary Reach r Frontispitce The rr.c.in1;ena.nce of: 100 illiie' long ;;;.pprocches to the purt o1' C<..lcutta • ... ,··er.g-.ge_d 'the· of the Authorities t:'lt, trl.dr.lle of ti'as .. - . a,ttimti~n . Po~t. sine~ t.:te 17tli ~ent.Jlrr·· -:co~seque·n~ on' the··~·.ncr~:ese of sizE" of th; v• a.1cl>HII c.i the:fi;., dr~fts J 'tn: PIV.bk,,m ':ci· ._i,~proving t~( '. navJ gabilit,.: ?f the d ver H::.o·;:tlj ·be cfl:U<o :n"re. .-.c•.-tb t h~ n in· olden C:a·rs. P .wt C'J·,, Ls l.l:".er:. .10'l:;ht. - _.; • ,. • I 't·ti~_ a,Ci:v~ce of vc;.z·iolls reccgnio~-d c-;,.:~rh.· · ~:1ey ~vere ~enerelly of th o~~i~J::,th;Ei~ the Jc:JD.e'' <,n::i !o:su li.eech was a bottleneck in the W/:J.Y of r.;oyi.~4ioz:O; 11',1 __ 1947,; $;rr (,laooe Ir::;lis, '1'41~·m the Port Coll1Dissioners con- ' . sulte~, advbe~: them to investig~te the meas•.re3 l"or i'::proving the navi- ~-. giiliili:ty .of the· river: i{o::>ghly by hydra'illc model studi·· s. proble:r: . T~ was> tharefore,_ :referred to the· CJr.trall\ater & Po~>.er Research Station - - - in Jlia,y 1947 •. -

Fluvial Geochemistry of Subarnarekha River Basin, India
J. Earth Syst. Sci. (2018) 127:119 c Indian Academy of Sciences https://doi.org/10.1007/s12040-018-1020-6 Fluvial geochemistry of Subarnarekha River basin, India Abhay Kumar Singh*, Soma Giri and Aaditya Chaturvedi Natural Resource and Environmental Management Division, CSIR – Central Institute of Mining and Fuel Research, Barwa Road, Dhanbad 826 015, India. *Corresponding author. e-mail: [email protected] MS received 25 July 2017; revised 30 January 2018; accepted 12 March 2018; published online 26 October 2018 The fluvial geochemistry of the Subarnarekha River and its major tributaries has been studied on a seasonal basis in order to assess the geochemical processes that explain the water composition and estimate solute fluxes. The analytical results show the mildly acidic to alkaline nature of the 2+ + − − Subarnarekha River water and the dominance of Ca and Na in cationic and HCO3 and Cl in anionic composition. Minimum ionic concentration during the monsoon and maximum concentration in the pre-monsoon seasons reflect concentrating effects due to decrease in the river discharge and increase in the base flow contribution during the pre-monsoon and dilution effects of atmospheric precipitation in the monsoon season. The solute acquisition processes are mainly controlled by weathering of rocks, with minor contribution from marine and anthropogenic sources. Higher contribution of alkaline earth (Ca2++Mg2+) to the total cations (TZ+) and high (Na+ +K+)/Cl−,(Na+ +K+)/TZ+, − 2− − 2+ 2+ + + HCO3 /(SO4 +Cl )andlow(Ca +Mg )/(Na +K ) equivalent ratios suggest that the Subarnarekha River water is under the combined influence of carbonate and silicate weathering. -

Types and Sources of Flood in Murshidabad, West Bengal
RESEARCH PAPER Science Volume : 3 | Issue : 2 | February 2013 | ISSN - 2249-555X Types and Sources of Flood in Murshidabad, West Bengal KEYWORDS Murshidabad, Types of Flood, Sources of Flood Swati Mollah Asst. Prof. in Geography, Department of Geography, Dumkal College, P.O. Basantapur, Dumkal, Murshidabad ABSTRACT Flood is defined as abnormal high stage of flow which overtops the natural or artificial river banks in any reach and causes immense loss of crops, property, human lives and lines of communication. It is a natural event that results from excess runoff generated from a drainage basin due to severe combination of critical hydrologic and meteorological condi- tions over the region. Murshidabad district is affected by frequent heavy flooding, drainage congestion and bank erosion, resulting in extensive submergence of land, loss of life and property and dislocation of the communication system. At times, the period of flood above danger level is 40 to 70 days. The present study aims to find out the main sources of occurrence of flood other than rainfall and their regional distribution. The paper identifies river bank erosion, overflow of western tributar- ies and discharge from dam, water logging from feeder canal, decay of spill channel and drainage congestion and overflow from the Ganga/Padma as the main sources of flood in Murshidabad. This study will help formulate of plans for flood man- agement according to the types of flood in the district and further detail study of sources of flood. INTRODUCTION es between 23˚43′- 24˚50′ N and 89˚49′- 88˚46′ E in cen- Two main rivers in Murshidabad are the Ganga-Padma and tral West Bengal.