The Spectacular Human Nose: an Amplifier of Individual Quality?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Evolutionary History of the Human Face
This is a repository copy of The evolutionary history of the human face. White Rose Research Online URL for this paper: https://eprints.whiterose.ac.uk/145560/ Version: Accepted Version Article: Lacruz, Rodrigo S, Stringer, Chris B, Kimbel, William H et al. (5 more authors) (2019) The evolutionary history of the human face. Nature Ecology and Evolution. pp. 726-736. ISSN 2397-334X https://doi.org/10.1038/s41559-019-0865-7 Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ THE EVOLUTIONARY HISTORY OF THE HUMAN FACE Rodrigo S. Lacruz1*, Chris B. Stringer2, William H. Kimbel3, Bernard Wood4, Katerina Harvati5, Paul O’Higgins6, Timothy G. Bromage7, Juan-Luis Arsuaga8 1* Department of Basic Science and Craniofacial Biology, New York University College of Dentistry; and NYCEP, New York, USA. 2 Department of Earth Sciences, Natural History Museum, London, UK 3 Institute of Human Origins and School of Human Evolution and Social Change, Arizona State University, Tempe, AZ. -

Effects of Glans Penis Augmentation Using Hyaluronic Acid Gel for Premature Ejaculation
International Journal of Impotence Research (2004) 16, 547–551 & 2004 Nature Publishing Group All rights reserved 0955-9930/04 $30.00 www.nature.com/ijir Effects of glans penis augmentation using hyaluronic acid gel for premature ejaculation JJ Kim1, TI Kwak1, BG Jeon1, J Cheon1 and DG Moon1* 1Department of Urology, Korea University College of Medicine, Sungbuk-ku, Seoul, Korea The main limitation of medical treatment for premature ejaculation is recurrence after withdrawal of medication. We evaluated the effect of glans penis augmentation using injectable hyaluronic acid (HA) gel for the treatment of premature ejaculation via blocking accessibility of tactile stimuli to nerve receptors. In 139 patients of premature ejaculation, dorsal neurectomy (Group I, n ¼ 25), dorsal neurectomy with glandular augmentation (Group II, n ¼ 49) and glandular augmentation (Group III, n ¼ 65) were carried out, respectively. Two branches of dorsal nerve preserving that of midline were cut at 2 cm proximal to coronal sulcus. For glandular augmentation, 2 cc of HA was injected into the glans penis, subcutaneously. At 6 months after each procedure, changes of glandular circumference were measured by tapeline in Groups II and III. In each groups, ejaculation time, patient’s satisfaction and partner’s satisfaction were also assessed. There was no significant difference in preoperative ejaculation time among three groups. Preoperative ejaculation times were 89.2740.29, 101.54759.42 and 96.5752.32 s in Groups I, II and III, respectively. Postoperative ejaculation times were significantly increased to 235.6758.6, 324.247107.58 and 281.9793.2 s in Groups I, II and III, respectively (Po0.01). -

Standard Human Facial Proportions
Name:_____________________________________________ Date:__________________Period: __________________ Standard Human Facial Proportions: The standard proportions for the human head can help you place facial features and find their orientation. The list below gives an idea of ideal proportions. • The eyes are halfway between the top of the head and the chin. • The face is divided into 3 parts from the hairline to the eyebrow, from the eyebrow to the bottom of the nose, and from the nose to the chin. • The bottom of the nose is halfway between the eyes and the chin. • The mouth is one third of the distance between the nose and the chin. • The distance between the eyes is equal to the width of one eye. • The face is about the width of five eyes and about the height of about seven eyes. • The base of the nose is about the width of the eye. • The mouth at rest is about the width of an eye. • The corners of the mouth line up with the centers of the eye. Their width is the distance between the pupils of the eye. • The top of the ears line up slightly above the eyes in line with the outer tips of the eyebrows. • The bottom of the ears line up with the bottom of the nose. • The width of the shoulders is equal to two head lengths. • The width of the neck is about ½ a head. Facial Feature Examples.docx Page 1 of 13 Name:_____________________________________________ Date:__________________Period: __________________ PROFILE FACIAL PROPORTIONS Facial Feature Examples.docx Page 2 of 13 Name:_____________________________________________ Date:__________________Period: -

How to Create the Perfect Eyebrow
Creating the Perfect Brows . As a make-up artist, I was acutely aware of the fact that my mother had a need that conventional cosmetics could not meet. She suffered from a type of alopecia (hair loss) of the eyebrows and lashes ever since I could remember. I would draw the eyebrows on for her, but it was often a painstaking process for her to draw the eyebrows on herself on a daily basis. After my mom described the embarrassment of swimming and having the pencil wash off her eyebrows, and another afternoon when a neighbor stopped by after she had been napping and one eyebrow was completely wiped off when she answered the door, it clearly was time for a solution. After completing my certification with the Academy of Micropigmentation, I had one of the most fulfilling days of my life, the day I was able to give a gift of eyebrows to my mother. This was a life changing experience for her and an incredible boost to her self-image. She went on to do her eyeliner, which was especially beneficial to her due to the absence of eyelashes, and I was able to give her lips a fuller appearance with the permanent lipliner. It is said that the facial feature models spend the most time on is their eyebrows and most women, at some point, have altered their eyebrows by tweezing, waxing or electrolysis. As women grow older, the hair on the outer half of their brows tend to become sparse or even non-existent. In ancient China, royalty were the only ones adorned with “tattooed” eyebrows. -

Clinical Review Nursingingeneralpractice
The health benefits of nose breathing Item Type Article Authors Allen, Ruth Publisher Nursing in General Practice Journal Nursing in General Practice Download date 01/10/2021 07:15:20 Link to Item http://hdl.handle.net/10147/559021 Find this and similar works at - http://www.lenus.ie/hse clinical review nursingingeneralpractice The health benefits of nose breathing DR Alan RUth, BehaviouRal Medicine PRactitioneR “For breath is life, and if you breathe well you will live long on earth.” sanskrit Proverb For the most part people are unaware of their breathing and take it for granted that they do it correctly. t has been estimated that approximately one third of people ing. However, it has been estimated that up to 30-50% of modern don’t breathe well enough to sustain normal health. These adults breathe through the mouth, especially during the early people do not get enough oxygenation of their cells, tissues morning hours. and organs. In the book Behavioural and Psychological Ap- Mouth breathing is common in individuals whose nasal proaches to Breathing Disorders, Dr Chandra Patel describes passages are blocked or restricted. A deviated nasal septum Ithe problem with breathing as follows: or small nostril size can lead a person to breathe through their “We start life with a breath, and the process continues mouth instead of their nose. However, breathing through the automatically for the rest of our lives. Because breathing mouth most of the time was not nature’s intention. Many studies continues on its own, without our awareness, it does not have demonstrated that chronic mouth breathing can result in a necessarily mean that it is always functioning for optimum number of adverse health consequences (see Table 1). -

Growth Hormone Deficiency Causing Micropenis:Peter A
Growth Hormone Deficiency Causing Micropenis:Peter A. Lee, MD, PhD, a Tom Mazur, PsyD, Lessons b Christopher P. Houk, MD, Learned c Robert M. Blizzard, MD d From a Well-Adjusted Adultabstract This report of a 46, XY patient born with a micropenis consistent with etiology from isolated congenital growth hormone deficiency is used to (1) raise the question regarding what degree testicular testosterone exposure aDepartment of Pediatrics, College of Medicine, Penn State to the central nervous system during fetal life and early infancy has on the University, Hershey, Pennsylvania; bCenter for Psychosexual development of male gender identity, regardless of gender of rearing; (2) Health, Jacobs School of Medicine and Biomedical suggest the obligatory nature of timely full disclosure of medical history; Sciences, University at Buffalo and John R. Oishei Children’s Hospital, Buffalo, New York; cDepartment of (3) emphasize that virtually all 46, XY infants with functional testes and Pediatrics, Medical College of Georgia, Augusta University, a micropenis should be initially boys except some with partial androgen Augusta, Georgia; and dDepartment of Pediatrics, College of Medicine, University of Virginia, Charlottesville, Virginia insensitivity syndrome; and (4) highlight the sustaining value of a positive long-term relationship with a trusted physician (R.M.B.). When this infant Dr Lee reviewed and discussed the extensive medical records with Dr Blizzard, reviewed presented, it was commonly considered inappropriate to gender assign an pertinent medical literature, and wrote each draft infant male whose penis was so small that an adult size was expected to be of the manuscript with input from all coauthors; inadequate, even if the karyotype was 46, XY, and testes were functional. -

Vivid Dreams/ Problems Sleeping: Nausea/Upset Stomach: Itching/Rash
Addressing NRT Barriers • Assess the severity of symptoms (Is it tolerable?). • Assess Hx: onset, duration and any troubleshooting that has already taken place. If indicated, get history of these symptoms when not taking these medications. You may also ask how Pt. would normally treat these symptoms. • If symptoms are tolerable àdevelop troubleshooting plan with Pt. Inform Pt. that many symptoms will go away after a few days. Reassess at next visit, but ask Pt. to call if symptoms persist/worsen or become intolerable before next call/visit. • If symptoms are not tolerableàconsider changing products or dosages as applicable. Consult study physician, as needed. Refer Pt. to their personal physician, if needed (e.g., prescription strength creams). • All potential cardiac symptoms should be promptly reported to study physician. Advise Pt. to discontinue NRT when indicated or instructed by study physician. Vivid Dreams/ Problems Sleeping: - Assess if sleep is being disrupted. Is night waking normal for Pt. – what is Pts.’ normal routine? - May try taking patch off at night, keeping in mind cravings may be stronger in the AM. After a couple of nights, try again to wear patch overnight. If using more than 1 patch, may consider only wearing 1 at night. - May try removing patch at night and putting on 2 hours before waking, especially when early morning waking is part of routine. Otherwise, can set an alarm, put on patch, and go back to sleep. - Regulate eating and sleeping patterns and use sleep hygiene tips (relaxation training, avoid caffeine). - Do not smoke or use short-acting NRT within 1-2 hours of bedtime (especially if sleep initiation is the major complaint.) Nausea/upset stomach: - Ask if nausea is only after using gum/lozenge or also after smoking a cigarette. -

Facial Image Comparison Feature List for Morphological Analysis
Disclaimer: As a condition to the use of this document and the information contained herein, the Facial Identification Scientific Working Group (FISWG) requests notification by e-mail before or contemporaneously to the introduction of this document, or any portion thereof, as a marked exhibit offered for or moved into evidence in any judicial, administrative, legislative, or adjudicatory hearing or other proceeding (including discovery proceedings) in the United States or any foreign country. Such notification shall include: 1) the formal name of the proceeding, including docket number or similar identifier; 2) the name and location of the body conducting the hearing or proceeding; and 3) the name, mailing address (if available) and contact information of the party offering or moving the document into evidence. Subsequent to the use of this document in a formal proceeding, it is requested that FISWG be notified as to its use and the outcome of the proceeding. Notifications should be sent to: Redistribution Policy: FISWG grants permission for redistribution and use of all publicly posted documents created by FISWG, provided the following conditions are met: Redistributions of documents, or parts of documents, must retain the FISWG cover page containing the disclaimer. Neither the name of FISWG, nor the names of its contributors, may be used to endorse or promote products derived from its documents. Any reference or quote from a FISWG document must include the version number (or creation date) of the document and mention if the document is in a draft status. Version 2.0 2018.09.11 Facial Image Comparison Feature List for Morphological Analysis 1. -

GLOSSARY of MEDICAL and ANATOMICAL TERMS
GLOSSARY of MEDICAL and ANATOMICAL TERMS Abbreviations: • A. Arabic • abb. = abbreviation • c. circa = about • F. French • adj. adjective • G. Greek • Ge. German • cf. compare • L. Latin • dim. = diminutive • OF. Old French • ( ) plural form in brackets A-band abb. of anisotropic band G. anisos = unequal + tropos = turning; meaning having not equal properties in every direction; transverse bands in living skeletal muscle which rotate the plane of polarised light, cf. I-band. Abbé, Ernst. 1840-1905. German physicist; mathematical analysis of optics as a basis for constructing better microscopes; devised oil immersion lens; Abbé condenser. absorption L. absorbere = to suck up. acervulus L. = sand, gritty; brain sand (cf. psammoma body). acetylcholine an ester of choline found in many tissue, synapses & neuromuscular junctions, where it is a neural transmitter. acetylcholinesterase enzyme at motor end-plate responsible for rapid destruction of acetylcholine, a neurotransmitter. acidophilic adj. L. acidus = sour + G. philein = to love; affinity for an acidic dye, such as eosin staining cytoplasmic proteins. acinus (-i) L. = a juicy berry, a grape; applied to small, rounded terminal secretory units of compound exocrine glands that have a small lumen (adj. acinar). acrosome G. akron = extremity + soma = body; head of spermatozoon. actin polymer protein filament found in the intracellular cytoskeleton, particularly in the thin (I-) bands of striated muscle. adenohypophysis G. ade = an acorn + hypophyses = an undergrowth; anterior lobe of hypophysis (cf. pituitary). adenoid G. " + -oeides = in form of; in the form of a gland, glandular; the pharyngeal tonsil. adipocyte L. adeps = fat (of an animal) + G. kytos = a container; cells responsible for storage and metabolism of lipids, found in white fat and brown fat. -

Empty Nose Syndrome
EMPTY NOSE SYNDROME 1 Empty Nose Syndrome 1.1 Definition The descriptive term “Empty nose syndrome” (ENS) was first coined by Kern and Stenkvist in 1994, who described the condition with substantial loss of tissue inside the nose in the region of the inferior and middle turbinates as “empty nose” (Scheithauer, 2010). “Empty nose” patients suffer from chronic nasal crusting, dryness, and intermittent bleeding. The most distinctive symptom of ENS is “paradoxical obstruction”- in that the patient suffers from difficulty breathing despite a widely patent nasal airway (Sozansky & Houser, 2014). ENS patients describe their feeling of nasal obstruction as constant and continuous: feeling of suffocation, inability or significant difficulty to breathe through their nose, feeling that their nose is too open, sensation of excessive airflow, shortness of breath, difficulty to properly inflate the lungs, lack of nasal resistance, and/or undifferentiated breathing difficulties (Houser, 2006; Houser, 2007; Chhabra & Houser, 2009; Scheithauer, 2010; Coste et al, 2012). 1.2 Primary symptoms ENS patients may experience various debilitating and devastating symptoms, in varying degrees, that can occur after excessive resection of functioning turbinate tissue from the nasal cavity (Moore & Kern, 2001). While the primary symptom is nasal airway obstruction, other symptoms depend upon the type of surgery, extent of functional nasal tissue removal, nature of the bacterial organisms growing inside the nasal cavity, and other unidentified factors. The set and level of severity of symptoms depends upon each individual case. ENS-related symptoms can be divided into three major categories and may include: Respiratory: paradoxical obstruction (feeling of suffocation, inability or significant difficulty to breathe through the nose, feeling that the nose is too open, sensation of excessive airflow, lack of nasal resistance, shortness of breath (dyspnea), difficulty to properly inflate the lungs, difficulty drawing a full breath, weakened airflow, and undifferentiated breathing difficulties) 1. -

1 the Ph of the Human Nail Plate S Murdan*, G
The pH of the human nail plate S Murdan*, G Milcovich and G S Goriparthi Department of Pharmaceutics, UCL School of Pharmacy, 29-39 Brunswick Square, London, WC1N 1AX, UK *corresponding author Tel: +44-2077535810; Fax: +44-2077535942; [email protected] Keywords: nail, pH, surface, tape stripping, washing, gender, acidity The work described in this paper was supported by The School of Pharmacy, University of London (now UCL School of Pharmacy). 1 ABSTRACT In this Chapter, measurements of the nailplate pH are reported. Measurements were conducted in vivo in 37 volunteers with healthy finger and toe nails, using a skin pH meter. The pH of unwashed and washed fingernails and the big toenails was measured and the influence of washing, anatomical site (fingers/toes), side (left/right), finger digit (digits 1-5) and gender were determined. The pH of the nail plate surface was around 5. There was no significant difference between the sides i.e. right or left hand/foot, among the ten fingernails, and between the two great toenails. However, toenails had a significantly higher pH than fingernails. Washing the nails caused an immediate, but transient increase in pH, which was not sustained with time, and pH returned to pre- washing levels within 20 minutes. In males, washing did not significantly influence finger or toe nailplate pH. In females however, washed fingernails had a significantly higher pH than unwashed ones, while there was no difference in the pH of the toenails. The pH of the nail plate interior, measured after tape-stripping, was found to be slightly lower than that at its surface. -
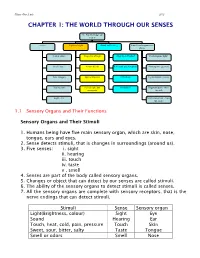
Chapter 1: the World Through Our Senses
Science Form 2 note 2012 CHAPTER 1: THE WORLD THROUGH OUR SENSES The World through our senses senses Light and sight Sound and hearing Stimuli and responses in plants Touch (skin) Properties of light Properties of sound Phototropism (light) Smell (nose) Vision defects Reflection and absorption Geotropism (gravity) Taste (tongue) Optical illusions limitations Hydrotropism (water) Hearing (ear) Stereoscopic and stereophonic Thigmotropism (move monocular toward) Sight (eye) Nastic movement (move run away) 1.1 Sensory Organs and Their Functions Sensory Organs and Their Stimuli 1. Humans being have five main sensory organ, which are skin, nose, tongue, ears and eyes. 2. Sense detects stimuli, that is changes in surroundings (around us). 3. Five senses: i. sight ii. hearing iii. touch iv. taste v . smell 4. Senses are part of the body called sensory organs. 5. Changes or object that can detect by our senses are called stimuli. 6. The ability of the sensory organs to detect stimuli is called senses. 7. All the sensory organs are complete with sensory receptors, that is the nerve endings that can detect stimuli. Stimuli Sense Sensory organ Light(Brightness, colour) Sight Eye Sound Hearing Ear Touch, heat, cold, pain, pressure Touch Skin Sweet, sour, bitter, salty Taste Tongue Smell or odors Smell Nose Science Form 2 note 2012 Laman web. http://freda.auyeung.net/5senses/see.htm http://freda.auyeung.net/5senses/touch.htm http://freda.auyeung.net/5senses/hear.htm http://freda.auyeung.net/5senses/taste.htm http://freda.auyeung.net/5senses/smell.htm 1.2 The Pathway from Stimulus to Response PMR 05 Stimulus Sensory organs Nerves Brain Nerve Response Figure 1.2 The summary of the pathway from stimulus to response 1.