Uplift-Driven Diversification in the Hengduan Mountains, a Temperate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Number 35 July-September
THE BULB NEWSLETTER Number 35 July-September 2001 Amana lives, long live Among! ln the Kew Scientist, Issue 19 (April 2001), Kew's Dr Mike Fay reports on the molecular work that has been carried out on Among. This little tulip«like eastern Asiatic group of Liliaceae that we have long grown and loved as Among (A. edulis, A. latifolla, A. erythroniolde ), but which took a trip into the genus Tulipa, should in fact be treated as a distinct genus. The report notes that "Molecular data have shown this group to be as distinct from Tulipa s.s. [i.e. in the strict sense, excluding Among] as Erythronium, and the three genera should be recognised.” This is good news all round. I need not change the labels on the pots (they still labelled Among), neither will i have to re~|abel all the as Erythronlum species tulips! _ Among edulis is a remarkably persistent little plant. The bulbs of it in the BN garden were acquired in the early 19605 but had been in cultivation well before that, brought back to England by a plant enthusiast participating in the Korean war. Although not as showy as the tulips, they are pleasing little bulbs with starry white flowers striped purplish-brown on the outside. It takes a fair amount of sun to encourage them to open, so in cool temperate gardens where the light intensity is poor in winter and spring, pot cultivation in a glasshouse is the best method of cultivation. With the extra protection and warmth, the flowers will open out almost flat. -

Quantifying Trends of Land Change in Qinghai-Tibet Plateau During 2001–2015
remote sensing Article Quantifying Trends of Land Change in Qinghai-Tibet Plateau during 2001–2015 Chao Wang, Qiong Gao and Mei Yu * Department of Environmental Sciences, University of Puerto Rico, Rio Piedras, San Juan, PR 00936, USA; [email protected] (C.W.); [email protected] (Q.G.) * Correspondence: [email protected]; Tel.: +1-787-764-0000 Received: 1 September 2019; Accepted: 17 October 2019; Published: 20 October 2019 Abstract: The Qinghai-Tibet Plateau (QTP) is among the most sensitive ecosystems to changes in global climate and human activities, and quantifying its consequent change in land-cover land-use (LCLU) is vital for assessing the responses and feedbacks of alpine ecosystems to global climate changes. In this study, we first classified annual LCLU maps from 2001–2015 in QTP from MODIS satellite images, then analyzed the patterns of regional hotspots with significant land changes across QTP, and finally, associated these trends in land change with climate forcing and human activities. The pattern of land changes suggested that forests and closed shrublands experienced substantial expansions in the southeastern mountainous region during 2001–2015 with the expansion of massive meadow loss. Agricultural land abandonment and the conversion by conservation policies existed in QTP, and the newly-reclaimed agricultural land partially offset the loss with the resulting net change of 5.1%. Although the urban area only expanded 586 km2, mainly at the expense of agricultural − land, its rate of change was the largest (41.2%). Surface water exhibited a large expansion of 5866 km2 (10.2%) in the endorheic basins, while mountain glaciers retreated 8894 km2 ( 3.4%) mainly in the − southern and southeastern QTP. -

Article-Lanzhou
13 by He Yuanqing, Yao Tandong, Cheng Guodong, and Yang Meixue Climatic records in a firn core from an Alpine temperate glacier on Mt. Yulong, southeastern part of the Tibetan Plateau Cold and Arid Regions Environmental and Engineering Research Institute, Chinese Academy of Sciences, Lanzhou 73000, China. Mt. Yulong is the southernmost glacier-covered area in Asian southwestern monsoon climate. Their total area is 11.61 km2. Eurasia, including China. There are 19 sub-tropical The glaciers resemble a group of flying dragons, giving Mt. Yulong temperate glaciers on the mountain, controlled by the (white dragon) as its name. Many explorers, tourists, poets and scientists have described southwestern monsoon climate. In the summer of 1999, Mt. Yulong from different points of view (Ward, 1924; Wissmann, a firn core, 10.10 m long, extending down to glacier ice, 1937). However, as they were unable to cross the extremely steep was recovered in the accumulation area of the largest mountain slopes and the forested area to the glacier above 4000 m a.s.l., some reported data were not correct. Most of the literature has glacier, Baishui No.1. Periodic variations of climatic focussed on the alpine landscape and the snow scenery, and there are signals above 7.8 m depth were apparent, and net accu- few accounts of the existing glaciers. Ren et al (1957) and Luo and mulation of four years was identified by the annual Yang (1963) first reported the distribution of these glaciers and of the late Pleistocene glacial landforms. To clarify the scale of glacia- oscillations of isotopic and ionic composition. -

Diversity and Geographical Pattern of Altitudinal Belts in the Hengduan Mountains in China
J. Mt. Sci. (2010) 7: 123–132 DOI: 10.1007/s11629-010-1011-9 Diversity and Geographical Pattern of Altitudinal Belts in the Hengduan Mountains in China YAO Yonghui, ZHANG Baiping*, HAN Fang, and PANG Yu Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, Beijing 100101, China. E-mail: [email protected] * Corresponding author, e-mail: [email protected] © Science Press and Institute of Mountain Hazards and Environment, CAS and Springer-Verlag Berlin Heidelberg 2010 Abstract: This paper analyses the diversity and effect needs further study. In addition, the data spatial pattern of the altitudinal belts in the quality and data accuracy at present also affect to Hengduan Mountains in China. A total of 7 types of some extent the result of quantitative modeling and base belts and 26 types of altitudinal belts are should be improved with RS/GIS in the future. identified in the study region. The main altitudinal belt lines, such as forest line, the upper limit of dark Keywords: Hengduan Mountains; Altitudinal belt coniferous forest and snow line, have similar spectra; Exposure effect; Quadratic model latitudinal and longitudinal spatial patterns, namely, arched quadratic curve model with latitudes and concave quadratic curve model along longitudinal Introduction direction. These patterns can be together called as “Hyperbolic-paraboloid model”, revealing the complexity and speciality of the environment and Globally and generally, the upper and lower ecology in the study region. This result further limits of altitudinal belts vary (increase) from high validates the hypnosis of a common quadratic model latitudes to low latitudes, from continental for spatial pattern of mountain altitudinal belts peripheries to inland areas, and from very humid proposed by the authors. -

Essd-2020-71.Pdf
Discussions https://doi.org/10.5194/essd-2020-71 Earth System Preprint. Discussion started: 7 September 2020 Science c Author(s) 2020. CC BY 4.0 License. Open Access Open Data 1 Consolidating the Randolph Glacier Inventory and the Glacier 2 Inventory of China over the Qinghai-Tibetan Plateau and th 3 Investigating Glacier Changes Since the mid-20 Century 4 Xiaowan Liu1,2,3, Zongxue Xu1,2, Hong Yang3,4, Xiuping Li5, Dingzhi Peng1,2 5 1College of Water Sciences, Beijing Normal University, Beijing, 100875, China 6 2Beijiing Key Laboratory of Urban Hydrological Cycle and Sponge City Technology, Beijing, 100875, China 7 3Eawag, Swiss Federal Institute of Aquatic Science and Technology, 8600 Dübendorf, Switzerland 8 4Department of Environmental Science, MGU University of Basel, Petersplatz 1, 4001, Switzerland 9 5Institute of Tibetan Plateau Research, Chinese Academy of Sciences, Beijing, 100101, China 10 Correspondence to: Zongxue Xu ([email protected]) 11 Abstract. Glacier retreat in the Qinghai-Tibetan Plateau (QTP), the ‘third pole of the world’, has attracted the 12 attention of researchers worldwide. Glacier inventories in the 1970s and the 2000s provide valuable information 13 to infer changes in individual glaciers. However, individual glacier volumes are either missing, incomplete or have 14 large errors in these inventories, and thus, the use of these datasets to investigate changes in glaciers in QTP in the 15 past few decades has become a challenge, particularly in the context of climate change. In this study, individual 16 glacier volume data in the Randolph Glacier Inventory version 4.0 (RGI 4.0, 1970s) and the second Glacier 17 Inventory of China (GIC-Ⅱ, 2000s) are recalculated and consolidated using a slope-dependent algorithm based on 18 elevation datasets for the QTP. -

Downloaded from Brill.Com10/07/2021 12:04:04PM Via Free Access © Koninklijke Brill NV, Leiden, 2017
Amphibia-Reptilia 38 (2017): 517-532 A new moth-preying alpine pit viper species from Qinghai-Tibetan Plateau (Viperidae, Crotalinae) Jingsong Shi1,2,∗, Gang Wang3, Xi’er Chen4, Yihao Fang5,LiDing6, Song Huang7,MianHou8,9, Jun Liu1,2, Pipeng Li9 Abstract. The Sanjiangyuan region of Qinghai-Tibetan Plateau is recognized as a biodiversity hotspot of alpine mammals but a barren area in terms of amphibians and reptiles. Here, we describe a new pit viper species, Gloydius rubromaculatus sp. n. Shi, Li and Liu, 2017 that was discovered in this region, with a brief taxonomic revision of the genus Gloydius.The new species can be distinguished from the other congeneric species by the following characteristics: cardinal crossbands on the back, indistinct canthus rostralis, glossy dorsal scales, colubrid-like oval head shape, irregular small black spots on the head scales, black eyes and high altitude distribution (3300-4770 m above sea level). The mitochondrial phylogenetic reconstruction supported the validity of the new species and furthermore reaffirms that G. intermedius changdaoensis, G. halys cognatus, G. h. caraganus and G. h. stejnegeri should be elevated as full species. Gloydius rubromaculatus sp. n. was found to be insectivorous: preying on moths (Lepidoptera, Noctuidae, Sideridis sp.) in the wild. This unusual diet may be one of the key factors to the survival of this species in such a harsh alpine environment. Keywords: Gloydius rubromaculatus sp. n., insectivorous, new species, Sanjiangyuan region. Introduction leopards (Uncia uncia), wild yaks (Bos grun- niens) and Tibetan antelopes (Pantholops hodg- The Sanjiangyuan region (the Source of Three sonii) (Shen and Tan, 2012). -
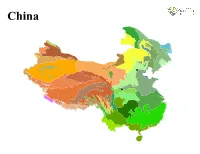
R Graphics Output
China China LEGEND Previously sampled Malaise trap site Ecoregion Alashan Plateau semi−desert North Tibetan Plateau−Kunlun Mountains alpine desert Altai alpine meadow and tundra Northeast China Plain deciduous forests Altai montane forest and forest steppe Northeast Himalayan subalpine conifer forests Altai steppe and semi−desert Northern Indochina subtropical forests Amur meadow steppe Northern Triangle subtropical forests Bohai Sea saline meadow Northwestern Himalayan alpine shrub and meadows Central China Loess Plateau mixed forests Nujiang Langcang Gorge alpine conifer and mixed forests Central Tibetan Plateau alpine steppe Ordos Plateau steppe Changbai Mountains mixed forests Pamir alpine desert and tundra Changjiang Plain evergreen forests Qaidam Basin semi−desert Da Hinggan−Dzhagdy Mountains conifer forests Qilian Mountains conifer forests Daba Mountains evergreen forests Qilian Mountains subalpine meadows Daurian forest steppe Qin Ling Mountains deciduous forests East Siberian taiga Qionglai−Minshan conifer forests Eastern Gobi desert steppe Rock and Ice Eastern Himalayan alpine shrub and meadows Sichuan Basin evergreen broadleaf forests Eastern Himalayan broadleaf forests South China−Vietnam subtropical evergreen forests Eastern Himalayan subalpine conifer forests Southeast Tibet shrublands and meadows Emin Valley steppe Southern Annamites montane rain forests Guizhou Plateau broadleaf and mixed forests Suiphun−Khanka meadows and forest meadows Hainan Island monsoon rain forests Taklimakan desert Helanshan montane conifer forests -

Evolutionary Events in Lilium (Including Nomocharis, Liliaceae
Molecular Phylogenetics and Evolution 68 (2013) 443–460 Contents lists available at SciVerse ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Evolutionary events in Lilium (including Nomocharis, Liliaceae) are temporally correlated with orogenies of the Q–T plateau and the Hengduan Mountains ⇑ Yun-Dong Gao a,b, AJ Harris c, Song-Dong Zhou a, Xing-Jin He a, a Key Laboratory of Bio-Resources and Eco-Environment of Ministry of Education, College of Life Science, Sichuan University, Chengdu 610065, China b Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China c Department of Botany, Oklahoma State University, Oklahoma 74078-3013, USA article info abstract Article history: The Hengduan Mountains (H-D Mountains) in China flank the eastern edge of the Qinghai–Tibet Plateau Received 21 July 2012 (Q–T Plateau) and are a center of great temperate plant diversity. The geological history and complex Revised 24 April 2013 topography of these mountains may have prompted the in situ evolution of many diverse and narrowly Accepted 26 April 2013 endemic species. Despite the importance of the H-D Mountains to biodiversity, many uncertainties Available online 9 May 2013 remain regarding the timing and tempo of their uplift. One hypothesis is that the Q–T Plateau underwent a final, rapid phase of uplift 8–7 million years ago (Mya) and that the H-D Mountains orogeny was a sep- Keywords: arate event occurring 4–3 Mya. To evaluate this hypothesis, we performed phylogenetic, biogeographic, Hengduan Mountains divergence time dating, and diversification rate analyses of the horticulturally important genus Lilium, Lilium–Nomocharis complex Intercontinental dispersal including Nomocharis. -

Long-Term Variations in Actual Evapotranspiration Over the Tibetan Plateau
Earth Syst. Sci. Data, 13, 3513–3524, 2021 https://doi.org/10.5194/essd-13-3513-2021 © Author(s) 2021. This work is distributed under the Creative Commons Attribution 4.0 License. Long-term variations in actual evapotranspiration over the Tibetan Plateau Cunbo Han1,2,3, Yaoming Ma1,2,4,5, Binbin Wang1,2, Lei Zhong6, Weiqiang Ma1,2,4, Xuelong Chen1,2,4, and Zhongbo Su7 1Land–Atmosphere Interaction and its Climatic Effects Group, State Key Laboratory of Tibetan Plateau Earth System and Resources Environment (TPESRE), Institute of Tibetan Plateau Research, Chinese Academy of Sciences, Beijing, China 2CAS Center for Excellence in Tibetan Plateau Earth Sciences, Chinese Academy of Sciences, Beijing, China 3Institute for Meteorology and Climate Research, Karlsruhe Institute of Technology, Karlsruhe, Germany 4College of Earth and Planetary Sciences, University of Chinese Academy of Sciences, Beijing, China 5Frontier Center for Eco-environment and Climate Change in Pan-third Pole Regions, Lanzhou University, Lanzhou, China 6Laboratory for Atmospheric Observation and Climate Environment Research, School of Earth and Space Sciences, University of Science and Technology of China, Hefei, China 7Faculty of Geo-Information Science and Earth Observation, University of Twente, Enschede, the Netherlands Correspondence: Y. Ma ([email protected]) Received: 30 October 2020 – Discussion started: 11 January 2021 Revised: 7 June 2021 – Accepted: 15 June 2021 – Published: 19 July 2021 Abstract. Actual terrestrial evapotranspiration (ETa) is a key parameter controlling land–atmosphere interac- tion processes and water cycle. However, spatial distribution and temporal changes in ETa over the Tibetan Plateau (TP) remain very uncertain. Here we estimate the multiyear (2001–2018) monthly ETa and its spatial distribution on the TP by a combination of meteorological data and satellite products. -

An Endemic Rat Species Complex Is Evidence of Moderate
www.nature.com/scientificreports OPEN An endemic rat species complex is evidence of moderate environmental changes in the Received: 14 June 2016 Accepted: 13 March 2017 terrestrial biodiversity centre of Published: 10 April 2017 China through the late Quaternary Deyan Ge1,*, Liang Lu2,*, Jilong Cheng1,3, Lin Xia1, Yongbin Chang1, Zhixin Wen1, Xue Lv1,3, Yuanbao Du1,3, Qiyong Liu2 & Qisen Yang1 The underlying mechanisms that allow the Hengduan Mountains (HDM), the terrestrial biodiversity centre of China, to harbour high levels of species diversity remain poorly understood. Here, we sought to explore the biogeographic history of the endemic rat, Niviventer andersoni species complex (NASC), and to understand the long-term persistence of high species diversity in this region. In contrast to previous studies that have proposed regional refuges in eastern or southern of the HDM and emphasized the influence of climatic oscillations on local vertebrates, we found that HDM as a whole acted as refuge for the NASC and that the historical range shifts of NASC mainly occurred in the marginal regions. Demographic analyses revealed slight recent population decline in Yunnan and south- eastern Tibet, whereas of the populations in Sichuan and of the entire NASC were stable. This pattern differs greatly from classic paradigms of temperate or alpine and holarctic species. Interestingly, the mean elevation, area and climate of potential habitats of clade a (N. excelsior), an alpine inhabitant, showed larger variations than did those of clade b (N. andersoni), a middle-high altitude inhabitant. These species represent the evolutionary history of montane small mammals in regions that were less affected by the Quaternary climatic changes. -

Endemic Wild Ornamental Plants from Northwestern Yunnan, China
HORTSCIENCE 40(6):1612–1619. 2005. have played an important role in world horti- culture and have been introduced to Western countries where they have been widely cul- Endemic Wild Ornamental Plants tivated. Some of the best known examples include Rhododendron, Primula, Gentiana, from Northwestern Yunnan, China Pedicularis, and Saussurea, which are all im- 1 portant genera in northwestern Yunnan (Chen Xiao-Xian Li and Zhe-Kun Zhou et al., 1989; Feng, 1983; Guan et al., 1998; Hu, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, P.R. 1990; Shi and Jin, 1999; Yang, 1956;). Many of China 650204 these ornamental species are endemic to small areas of northwestern Yunnan (e.g., Rhododen- Additional index words. horticultural potential dron russatum), therefore, their cultivation not Abstract. Northwestern Yunnan is situated in the southern part of the Hengduan Mountains, only provides for potential sources of income which is a complex and varied natural environment. Consequently, this region supports a generation, but also offers a potential form of great diversity of endemic plants. Using fi eld investigation in combination with analysis conservation management: these plants can of relevant literature and available data, this paper presents a regional ethnobotanical be used directly for their ornamental plant study of this area. Results indicated that northwestern Yunnan has an abundance of wild value or as genetic resources for plant breed- ornamental plants: this study identifi ed 262 endemic species (belonging to 64 genera and ing programs. The aims of current paper are 28 families) with potential ornamental value. The distinguishing features of these wild to describe the unique fl ora of northwestern plants, their characteristics and habitats are analyzed; the ornamental potential of most Yunnan and provide detailed information of plants stems from their wildfl owers, but some species also have ornamental fruits and those resources, in terms of their potential foliage. -

Phylogeography of Prunus Armeniaca L. Revealed by Chloroplast DNA And
www.nature.com/scientificreports OPEN Phylogeography of Prunus armeniaca L. revealed by chloroplast DNA and nuclear ribosomal sequences Wen‑Wen Li1, Li‑Qiang Liu1, Qiu‑Ping Zhang2, Wei‑Quan Zhou1, Guo‑Quan Fan3 & Kang Liao1* To clarify the phytogeography of Prunus armeniaca L., two chloroplast DNA fragments (trnL‑trnF and ycf1) and the nuclear ribosomal DNA internal transcribed spacer (ITS) were employed to assess genetic variation across 12 P. armeniaca populations. The results of cpDNA and ITS sequence data analysis showed a high the level of genetic diversity (cpDNA: HT = 0.499; ITS: HT = 0.876) and a low level of genetic diferentiation (cpDNA: FST = 0.1628; ITS: FST = 0.0297) in P. armeniaca. Analysis of molecular variance (AMOVA) revealed that most of the genetic variation in P. armeniaca occurred among individuals within populations. The value of interpopulation diferentiation (NST) was signifcantly higher than the number of substitution types (GST), indicating genealogical structure in P. armeniaca. P. armeniaca shared genotypes with related species and may be associated with them through continuous and extensive gene fow. The haplotypes/genotypes of cultivated apricot populations in Xinjiang, North China, and foreign apricot populations were mixed with large numbers of haplotypes/ genotypes of wild apricot populations from the Ili River Valley. The wild apricot populations in the Ili River Valley contained the ancestral haplotypes/genotypes with the highest genetic diversity and were located in an area considered a potential glacial refugium for P. armeniaca. Since population expansion occurred 16.53 kyr ago, the area has provided a suitable climate for the population and protected the genetic diversity of P.