Embryonic and Larval Development of Spisula (Bivalvia: Mactridae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spisula Subtruncata (Da Costa, 1778)
Spisula subtruncata (da Costa, 1778) AphiaID: 140302 AMEIJOA Animalia (Reino) > Mollusca (Filo) > Bivalvia (Classe) > Autobranchia (Subclasse) > Heteroconchia (Infraclasse) > Imparidentia (Superordem) > Venerida (Ordem) > Mactroidea (Superfamilia) > Mactridae (Familia) Rainer Borcherding - Schutzstation Wattenmeer, via beachexplorer.org Kirsten Thiemann, via beachexplorer.org Principais ameaças Sinónimos Mactra deltoides Lamarck, 1818 Mactra euxinica Krynicki, 1837 Mactra lactea Poli, 1791 Mactra striata T. Brown, 1827 Mactra subtruncata (da Costa, 1778) 1 Mactra subtruncata var. conemenosi Bucquoy, Dautzenberg & Dollfus, 1896 Mactra subtruncata var. inaequalis Jeffreys, 1864 Mactra subtruncata var. tenuis Jeffreys, 1864 Mactra subtruncata var. transversa Pallary, 1902 Mactra triangula Brocchi, 1814 Spisula triangula (Brocchi, 1814) Trigonella subtruncata da Costa, 1778 Referências additional source Howson, C. M.; Picton, B. E. (1997). The species directory of the marine fauna and flora of the British Isles and surrounding seas. Ulster Museum Publication, 276. The Ulster Museum: Belfast, UK. ISBN 0-948150-06-8. vi, 508 (+ cd-rom) pp. [details] basis of record Gofas, S.; Le Renard, J.; Bouchet, P. (2001). Mollusca. in: Costello, M.J. et al. (eds), European Register of Marine Species: a check-list of the marine species in Europe and a bibliography of guides to their identification. Patrimoines Naturels. 50: 180-213. [details] additional source Huber, M. (2010). Compendium of bivalves. A full-color guide to 3,300 of the world’s marine bivalves. A status on Bivalvia after 250 years of research. Hackenheim: ConchBooks. 901 pp., 1 CD-ROM. [details] context source (Schelde) Maris, T.; Beauchard, O.; Van Damme, S.; Van den Bergh, E.; Wijnhoven, S.; Meire, P. (2013). Referentiematrices en Ecotoopoppervlaktes Annex bij de Evaluatiemethodiek Schelde-estuarium Studie naar “Ecotoopoppervlaktes en intactness index”. -

Zhang Et Al., 2015
Estuarine, Coastal and Shelf Science 153 (2015) 38e53 Contents lists available at ScienceDirect Estuarine, Coastal and Shelf Science journal homepage: www.elsevier.com/locate/ecss Modeling larval connectivity of the Atlantic surfclams within the Middle Atlantic Bight: Model development, larval dispersal and metapopulation connectivity * Xinzhong Zhang a, , Dale Haidvogel a, Daphne Munroe b, Eric N. Powell c, John Klinck d, Roger Mann e, Frederic S. Castruccio a, 1 a Institute of Marine and Coastal Science, Rutgers University, New Brunswick, NJ 08901, USA b Haskin Shellfish Research Laboratory, Rutgers University, Port Norris, NJ 08349, USA c Gulf Coast Research Laboratory, University of Southern Mississippi, Ocean Springs, MS 39564, USA d Center for Coastal Physical Oceanography, Old Dominion University, Norfolk, VA 23529, USA e Virginia Institute of Marine Science, The College of William and Mary, Gloucester Point, VA 23062, USA article info abstract Article history: To study the primary larval transport pathways and inter-population connectivity patterns of the Atlantic Received 19 February 2014 surfclam, Spisula solidissima, a coupled modeling system combining a physical circulation model of the Accepted 30 November 2014 Middle Atlantic Bight (MAB), Georges Bank (GBK) and the Gulf of Maine (GoM), and an individual-based Available online 10 December 2014 surfclam larval model was implemented, validated and applied. Model validation shows that the model can reproduce the observed physical circulation patterns and surface and bottom water temperature, and Keywords: recreates the observed distributions of surfclam larvae during upwelling and downwelling events. The surfclam (Spisula solidissima) model results show a typical along-shore connectivity pattern from the northeast to the southwest individual-based model larval transport among the surfclam populations distributed from Georges Bank west and south along the MAB shelf. -

Spatial Variability in Recruitment of an Infaunal Bivalve
Spatial Variability in Recruitment of an Infaunal Bivalve: Experimental Effects of Predator Exclusion on the Softshell Clam (Mya arenaria L.) along Three Tidal Estuaries in Southern Maine, USA Author(s): Brian F. Beal, Chad R. Coffin, Sara F. Randall, Clint A. Goodenow Jr., Kyle E. Pepperman, Bennett W. Ellis, Cody B. Jourdet and George C. Protopopescu Source: Journal of Shellfish Research, 37(1):1-27. Published By: National Shellfisheries Association https://doi.org/10.2983/035.037.0101 URL: http://www.bioone.org/doi/full/10.2983/035.037.0101 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Journal of Shellfish Research, Vol. 37, No. 1, 1–27, 2018. SPATIAL VARIABILITY IN RECRUITMENT OF AN INFAUNAL BIVALVE: EXPERIMENTAL EFFECTS OF PREDATOR EXCLUSION ON THE SOFTSHELL CLAM (MYA ARENARIA L.) ALONG THREE TIDAL ESTUARIES IN SOUTHERN MAINE, USA 1,2 3 2 3 BRIAN F. -

TREATISE ONLINE Number 48
TREATISE ONLINE Number 48 Part N, Revised, Volume 1, Chapter 31: Illustrated Glossary of the Bivalvia Joseph G. Carter, Peter J. Harries, Nikolaus Malchus, André F. Sartori, Laurie C. Anderson, Rüdiger Bieler, Arthur E. Bogan, Eugene V. Coan, John C. W. Cope, Simon M. Cragg, José R. García-March, Jørgen Hylleberg, Patricia Kelley, Karl Kleemann, Jiří Kříž, Christopher McRoberts, Paula M. Mikkelsen, John Pojeta, Jr., Peter W. Skelton, Ilya Tëmkin, Thomas Yancey, and Alexandra Zieritz 2012 Lawrence, Kansas, USA ISSN 2153-4012 (online) paleo.ku.edu/treatiseonline PART N, REVISED, VOLUME 1, CHAPTER 31: ILLUSTRATED GLOSSARY OF THE BIVALVIA JOSEPH G. CARTER,1 PETER J. HARRIES,2 NIKOLAUS MALCHUS,3 ANDRÉ F. SARTORI,4 LAURIE C. ANDERSON,5 RÜDIGER BIELER,6 ARTHUR E. BOGAN,7 EUGENE V. COAN,8 JOHN C. W. COPE,9 SIMON M. CRAgg,10 JOSÉ R. GARCÍA-MARCH,11 JØRGEN HYLLEBERG,12 PATRICIA KELLEY,13 KARL KLEEMAnn,14 JIřÍ KřÍž,15 CHRISTOPHER MCROBERTS,16 PAULA M. MIKKELSEN,17 JOHN POJETA, JR.,18 PETER W. SKELTON,19 ILYA TËMKIN,20 THOMAS YAncEY,21 and ALEXANDRA ZIERITZ22 [1University of North Carolina, Chapel Hill, USA, [email protected]; 2University of South Florida, Tampa, USA, [email protected], [email protected]; 3Institut Català de Paleontologia (ICP), Catalunya, Spain, [email protected], [email protected]; 4Field Museum of Natural History, Chicago, USA, [email protected]; 5South Dakota School of Mines and Technology, Rapid City, [email protected]; 6Field Museum of Natural History, Chicago, USA, [email protected]; 7North -

Gametogenic Cycle of <I>Rangia Cuneata</I> (Mactridae, Mollusca
BULLETIN OF MARINE SCIENCE, 45(1): 130-138, 1989 GAMETOGENIC CYCLE OF RANGIA CUNEATA (MACTRIDAE, MOLLUSCA) IN MOBILE BAY, ALABAMA, WITH COMMENTS ON GEOGRAPHIC VARIATION M. C. Jovanovich and K. R. Marion ABSTRACT Stages of the gametogenic cycle of the brackish water clam Rangia cuneata were investigated in Mobile Bay, Alabama, by histological examination of the gonads. Water temperature and salinity measurements were related to the gametogenic cycle and compared to those made in other studies on the same species in different locations. Clams in the early active phase were found from February through April, and those in the late active phase predominated in May. Gonads were ripe from July through September and were partially spawned from September through October. Clams became spent between November and January. Changes in meat, gonad, and total wet weight reflected the stages of the gametogenic cycle, while length of the shells remained nearly constant year round. The gametogenic cycle of R. cuneata in Mobile Bay followed a similar pattern to that observed in clams studied in other locations, except that gametogenesis began earlier, during late winter, and clams became spent earlier in the fall. It is suggested that the interactive effects of temperature, salinity, and nutrients can account for the differences in the timing and length of the stages of the gametogenic cycle between locations. Rangia cuneata (Gray) is a brackish water clam (Family Mactridae) abundant in the estuaries of the Gulf of Mexico. It is also found throughout the coastal areas of the eastern United States as far north as the upper Chesapeake Bay (Woodburn, 1962; Pfitzenmeyer, 1970). -

Surfclam Aquaculture Techniq
Final Report Piloting Surf Clam Aquaculture Techniques to Create Commercial Opportunities Award Number: NA16NMF4270241 Award Period: 03/01/2017 – 02/28/2020 Recipient Name: Aquacultural Research Corporation (dba A.R.C. Hatchery) Program Office: Fisheries Headquarters Program Office (FHQ) Program Officer: Deirdre Kimball, 978-281-9290, [email protected] Project Title: Piloting Surf Clam Aquaculture Techniques to Create Commercial Opportunities PIs/PDs: Rick Sawyer Partners: Cape Cod Cooperative Extension/Woods Hole Sea Grant, Cape Cod Commercial Fishermen’s Alliance, Roger Williams University Report Type: Performance Final Report Reporting Period: 03/01/2017 – 02/28/2020 Final Report: Yes Report Due Date: 08/27/2020 1 TABLE OF CONTENTS ACRONYMS/DEFINITIONS ..................................................................................................................4 EXECUTIVE SUMMARY .......................................................................................................................5 PURPOSE ...........................................................................................................................................8 BACKGROUND .............................................................................................................................................. 8 MARKET OPPORTUNITY .................................................................................................................................. 9 IMPORTANCE OF DEVELOPING THIS NEW SPECIES ............................................................................................ -

Palaeodemecological Analysis of Infaunal Bivalves “Lebensspuren” from the Mulichinco Formation, Lower Cretaceous, Neuquén Basin, Argentina
AMEGHINIANA - 2012 - Tomo 49 (1): 47 – 59 ISSN 0002-7014 PALAEODEMECOLOGICAL ANALYSIS OF INFAUNAL BIVALVES “LEBENSSPUREN” FROM THE MULICHINCO FORMATION, LOWER CRETACEOUS, NEUQUÉN BASIN, ARGENTINA JAVIER ECHEVARRÍA, SUSANA E. DAMBORENEA and MIGUEL O. MANCEÑIDO División Paleozoología Invertebrados, Museo de La Plata, Paseo del Bosque s/n, B1900FWA La Plata, Argentina - Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). [email protected] Abstract. The study of palaeodemecological features requires some particular taphonomic conditions. These conditions were met in the Mulichinco Formation (Valanginian), where burrowing bivalve trace fossils are widespread and often appear in cross section on bedding surfaces. Two groups of such beds were analyzed, measuring population density, spatial distribution, size distribution and horizontal orienta- tion of the burrows. The palaeoenvironment was established by means of a detailed sedimentological analysis, and the bivalve fauna present was checked, in order to attempt identifying their potential producers. High population densities were found in the two groups, indicating favourable physical conditions and good food supply, while differences in both spatial and size distributions were noticed between them; on most surfaces there was no preferred orientation. The first group (group A) showed a uniform pattern of spatial distribution and larger traces, with a remarkable absence of small sizes. In the second group (group B), the spatial distribution pattern is indistinguishable from a random distribution (except one case in which the pattern appears to be aggregated). Group A is interpreted as a set of escape traces made by deep burrowers in response to storm deposition, while group B is considered as resting/escape traces made by shallow burrowers in tide-dominated environments. -

The Zebra Mussel in Europe“
Chapter 2 NeogeNe dreisseNids iN CeNtral europe: evolutioNary shifts aNd diversity ChaNges Mathias Harzhauser and Oleg Mandic This chapter was originally published in the book „The Zebra Mussel in Europe“. The copy attached is provided by Margraf Publishers GmbH for the author‘s benefit and for the benefit of the author‘s institution for non-commercial research and educational use. All other uses, reproduction and distribution are prohibited and require a written permission by the publisher. G. van der Velde, S. Rajagopal & A. bij de Vaate de bij A. & Rajagopal S. Velde, der van G. The Zebra Mussel in Europe THE ZEBRA MUSSEL IN EUROPE Europe in Mussel Zebra The Edited by Gerard van der Velde, Sanjeevi Rajagopal & Abraham bij de Vaate The Zebra mussel (Dreissena polymorpha) is one of world’s most successful invasive species. Originating from the Ponto-Caspian region, it spread all over Europe and crossed over to North America via the ocean. Wherever it has spread, it made its presence felt with tremendous ecological and economic impact. To make matters worse, the species is still expanding its geographical range. Although there is a stream of information concerning the zebra mussel and its relatives, a recent up-to-date book summarizing the newest information on the zebra mussel was lacking. The present book is expected to fill this gap. It deals with edited by all aspects of the zebra mussel and some of its relatives, varying in its content from Gerard van der Velde taxonomy and phylogeny, to fossil and recent species, distribution and dispersal, genetics, food, growth and life history, ecology and ecological impact, endosymbionts, parasites, Sanjeevi Rajagopal predation, use as indicator for water quality and for water quality improvement and biofouling Abraham bij de Vaate and control. -

The Evolution of Extreme Longevity in Modern and Fossil Bivalves
Syracuse University SURFACE Dissertations - ALL SURFACE August 2016 The evolution of extreme longevity in modern and fossil bivalves David Kelton Moss Syracuse University Follow this and additional works at: https://surface.syr.edu/etd Part of the Physical Sciences and Mathematics Commons Recommended Citation Moss, David Kelton, "The evolution of extreme longevity in modern and fossil bivalves" (2016). Dissertations - ALL. 662. https://surface.syr.edu/etd/662 This Dissertation is brought to you for free and open access by the SURFACE at SURFACE. It has been accepted for inclusion in Dissertations - ALL by an authorized administrator of SURFACE. For more information, please contact [email protected]. Abstract: The factors involved in promoting long life are extremely intriguing from a human perspective. In part by confronting our own mortality, we have a desire to understand why some organisms live for centuries and others only a matter of days or weeks. What are the factors involved in promoting long life? Not only are questions of lifespan significant from a human perspective, but they are also important from a paleontological one. Most studies of evolution in the fossil record examine changes in the size and the shape of organisms through time. Size and shape are in part a function of life history parameters like lifespan and growth rate, but so far little work has been done on either in the fossil record. The shells of bivavled mollusks may provide an avenue to do just that. Bivalves, much like trees, record their size at each year of life in their shells. In other words, bivalve shells record not only lifespan, but also growth rate. -
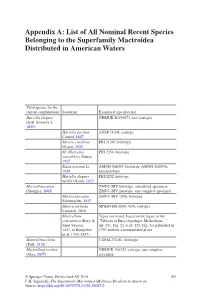
List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters
Appendix A: List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters Valid species (in the current combination) Synonym Examined type material Harvella elegans NHMUK 20190673, two syntypes (G.B. Sowerby I, 1825) Harvella pacifica ANSP 51308, syntype Conrad, 1867 Mactra estrellana PRI 21265, holotype Olsson, 1922 M. (Harvella) PRI 2354, holotype sanctiblasii Maury, 1925 Raeta maxima Li, AMNH 268093, lectotype; AMNH 268093a, 1930 paralectotype Harvella elegans PRI 2252, holotype tucilla Olsson, 1932 Mactrellona alata ZMUC-BIV, holotype, articulated specimen; (Spengler, 1802) ZMUC-BIV, paratype, one complete specimen Mactra laevigata ZMUC-BIV 1036, holotype Schumacher, 1817 Mactra carinata MNHN-IM-2000-7038, syntypes Lamarck, 1818 Mactrellona Types not found, based on the figure of the concentrica (Bory de “Tableau of Encyclopedique Methodique…” Saint Vincent, (pl. 251, Fig. 2a, b, pl. 252, Fig. 2c) published in 1827, in Bruguière 1797 without a nomenclatorial act et al. 1791–1827) Mactrellona clisia USNM 271481, holotype (Dall, 1915) Mactrellona exoleta NHMUK 196327, syntype, one complete (Gray, 1837) specimen © Springer Nature Switzerland AG 2019 103 J. H. Signorelli, The Superfamily Mactroidea (Mollusca:Bivalvia) in American Waters, https://doi.org/10.1007/978-3-030-29097-9 104 Appendix A: List of All Nominal Recent Species Belonging to the Superfamily… Valid species (in the current combination) Synonym Examined type material Lutraria ventricosa MCZ 169451, holotype; MCZ 169452, paratype; -

Growth Estimations of the Argentinean Wedge Clam Donax Hanleyanus: a Comparison Between Length-Frequency Distribution and Size-Increment Analysis
Journal of Experimental Marine Biology and Ecology 379 (2009) 8–15 Contents lists available at ScienceDirect Journal of Experimental Marine Biology and Ecology journal homepage: www.elsevier.com/locate/jembe Growth estimations of the Argentinean wedge clam Donax hanleyanus: A comparison between length-frequency distribution and size-increment analysis Marko Herrmann a,b,⁎, Mauro L. Lepore b, Jürgen Laudien a, Wolf E. Arntz a, Pablo E. Penchaszadeh b a Alfred Wegener Institute for Polar and Marine Research, Section of Marine Animal Ecology, P.O. Box 120161, 27515 Bremerhaven, Germany b Museo Argentino de Ciencias Naturales, Av. Angel Gallardo 470 3° piso lab. 80, C1405DJR Buenos Aires, Argentina article info abstract Article history: Growth rates of the Argentinean wedge clam Donax hanleyanus were estimated comparing two different Received 19 August 2008 methods in the intertidal of the exposed sandy beach Mar de las Pampas: (i) results of a relatively shortly Received in revised form 19 July 2009 (49 days) tagging-recapture experiment using the in situ fluorescent marking (IFM) method and subsequent Accepted 23 July 2009 size-increment analyses were compared with results from (ii) length-frequency distributions (LFD) analysis originating from a time consuming 25 month quantitative sampling. Residuals, derived from IFM method Keywords: and LFD analysis, were of similar magnitude and distribution, indicating that both methods are equally Calcein appropriate to estimate growth of D. hanleyanus. Comparing overall growth performance indices (OGPs) of Comparison of methods Daily growth rate several Donax species from different climate areas it resulted that growth of temperate bivalves can be In situ fluorescent marking estimated well by carrying out a relatively short-time tagging-recapture experiment using IFM but it is In vitro suitability of stains recommended to use both, the IFM as well as the LFD method to determine growth of tropical bivalves. -

Fossil Bivalves and the Sclerochronological Reawakening
Paleobiology, 2021, pp. 1–23 DOI: 10.1017/pab.2021.16 Review Fossil bivalves and the sclerochronological reawakening David K. Moss* , Linda C. Ivany, and Douglas S. Jones Abstract.—The field of sclerochronology has long been known to paleobiologists. Yet, despite the central role of growth rate, age, and body size in questions related to macroevolution and evolutionary ecology, these types of studies and the data they produce have received only episodic attention from paleobiologists since the field’s inception in the 1960s. It is time to reconsider their potential. Not only can sclerochrono- logical data help to address long-standing questions in paleobiology, but they can also bring to light new questions that would otherwise have been impossible to address. For example, growth rate and life-span data, the very data afforded by chronological growth increments, are essential to answer questions related not only to heterochrony and hence evolutionary mechanisms, but also to body size and organism ener- getics across the Phanerozoic. While numerous fossil organisms have accretionary skeletons, bivalves offer perhaps one of the most tangible and intriguing pathways forward, because they exhibit clear, typically annual, growth increments and they include some of the longest-lived, non-colonial animals on the planet. In addition to their longevity, modern bivalves also show a latitudinal gradient of increasing life span and decreasing growth rate with latitude that might be related to the latitudinal diversity gradient. Is this a recently developed phenomenon or has it characterized much of the group’s history? When and how did extreme longevity evolve in the Bivalvia? What insights can the growth increments of fossil bivalves provide about hypotheses for energetics through time? In spite of the relative ease with which the tools of sclerochronology can be applied to these questions, paleobiologists have been slow to adopt sclerochrono- logical approaches.