Seed Availability Assessment and Seed Collection of Wild Plants in Selabintana Forest, Mount Gede Pangrango National Park, West Java
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acronychia Acidula Click on Images to Enlarge
Species information Abo ut Reso urces Hom e A B C D E F G H I J K L M N O P Q R S T U V W X Y Z Acronychia acidula Click on images to enlarge Family Rutaceae Scientific Name Acronychia acidula F.Muell. Fruit, several views, cross sections and seeds. Copyright W. Mueller, F.J.H. von (1864) Fragmenta Phytographiae Australiae 4: 154. Type: In montibus Seaview Range T. Cooper apud Rockingham Bay. Dallachy. Common name Hard Aspen; Lemon Aspen; Lemon Wood Stem Blaze odour generally conspicuous, difficult to describe, but perhaps resembling mango (Mangifera indica) or citrus (Citrus spp.). Leaves Leaves and Flowers. Copyright B. Gray Leaf blades about 10.5-19.5 x 5-11 cm. About 8-20 main lateral veins on each side of the midrib. Underside of the leaf blade only slightly paler than the upper surface. Crushed leaves often emit an odour like mango skin (Mangifera indica). Flowers Inflorescence usually more than 2 cm long. Flowers about 9.5 mm long. Stamens eight, dimorphic, four long and four short, in one whorl, long and short stamens alternating. Disk and style yellow. Ovary and stigma green. Fruit Scale bar 10mm. Copyright CSIRO Fruits +/- globular, about 20 mm diam. Seeds about 4.5 mm long. Seedlings Cotyledons about 9-11 mm long, margins toothed. First and second pairs of leaves trifoliolate. At the tenth leaf stage: leaf blade inconspicuously toothed, more conspicuous on earlier leaves. Seed germination time 51 to 226 days. Distribution and Ecology Endemic to Queensland, occurs in NEQ and CEQ. -

Phylogeny and Classification of the Melastomataceae and Memecylaceae
Nord. J. Bot. - Section of tropical taxonomy Phylogeny and classification of the Melastomataceae and Memecy laceae Susanne S. Renner Renner, S. S. 1993. Phylogeny and classification of the Melastomataceae and Memecy- laceae. - Nord. J. Bot. 13: 519-540. Copenhagen. ISSN 0107-055X. A systematic analysis of the Melastomataceae, a pantropical family of about 4200- 4500 species in c. 166 genera, and their traditional allies, the Memecylaceae, with c. 430 species in six genera, suggests a phylogeny in which there are two major lineages in the Melastomataceae and a clearly distinct Memecylaceae. Melastomataceae have close affinities with Crypteroniaceae and Lythraceae, while Memecylaceae seem closer to Myrtaceae, all of which were considered as possible outgroups, but sister group relationships in this plexus could not be resolved. Based on an analysis of all morph- ological and anatomical characters useful for higher level grouping in the Melastoma- taceae and Memecylaceae a cladistic analysis of the evolutionary relationships of the tribes of the Melastomataceae was performed, employing part of the ingroup as outgroup. Using 7 of the 21 characters scored for all genera, the maximum parsimony program PAUP in an exhaustive search found four 8-step trees with a consistency index of 0.86. Because of the limited number of characters used and the uncertain monophyly of some of the tribes, however, all presented phylogenetic hypotheses are weak. A synapomorphy of the Memecylaceae is the presence of a dorsal terpenoid-producing connective gland, a synapomorphy of the Melastomataceae is the perfectly acrodro- mous leaf venation. Within the Melastomataceae, a basal monophyletic group consists of the Kibessioideae (Prernandra) characterized by fiber tracheids, radially and axially included phloem, and median-parietal placentation (placentas along the mid-veins of the locule walls). -
Pollination, Mating System, Phenology and Characterisation of Medinilla Multiflora Merr
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/329683248 Pollination, Mating System, Phenology and Characterisation of Medinilla multiflora Merr. (Melastomataceae) on Mt. Makiling, Philippines Article · July 2018 DOI: 10.24823/Sibbaldia.2018.251 CITATION READS 1 582 1 author: J. Peter Quakenbush University of the Philippines Los Baños 3 PUBLICATIONS 9 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Medinilla View project All content following this page was uploaded by J. Peter Quakenbush on 15 December 2018. The user has requested enhancement of the downloaded file. SIBBALDIA: 121 The Journal of Botanic Garden Horticulture, No. 16 POLLINATION, MATING SYSTEM, PHENOLOGY AND CHARACTERISATION OF MEDINILLA MULTIFLORA MERR. (MELASTOMATACEAE) ON MT MAKILING, PHILIPPINES J. Peter Quakenbush1 ABSTRACT An investigation into the reproductive biology of Medinilla multiflora Merr. (Melastomataceae) from Mt Makiling, Luzon, is presented. This includes a morphological and distributional exami- nation of the population on the mountain, the documentation of reproductive phenological patterns, a study of the mating system and observations of biotic interactions. Measurements were made of trait variability, reproductive phenology was characterised from field and herbarium observations, stigmatic receptivity was tested by counting pollen germination, insect exclusion and hand-pollination experiments helped determine the mating system and field observations recorded the identity and behaviour of floral visitors. Significant reproductive morphological differences were found between described populations. This identified a need for the recognition of this diversity and further delimitation of the Medinilla multiflora species complex. Although Medinilla multiflora produced flowers and fruit year-round, the population also exhibited cycles of increased reproduction most likely initiated by seasonal low temperatures. -

Detailed Final Report
An urgent conservation call from endemic plants of Mount Salak, West Java, Indonesia I Robiansyah* and S U Rakhmawati Research Center for Plant Conservation and Botanic Gardens - LIPI. Jl.Ir.H. Juanda 13 Bogor 16003, West Java, Indonesia *[email protected] Abstract. Mount Salak is part of Mount Halimun-Salak National Park in West Java, Indonesia. It is home to five endemic plant species that are very susceptible to human interference due to their close proximity to human settlements. The deforestation rate of the area was 1,473 ha or 1.3% of the total area each year. Using eleven line transects with a total length of 44.76 km, the present study aims at providing data on current population and conservation status of these five endemic plant species. The results showed that there was an urgent conservation call from Mount Salak as all five targeted species were unable to be located. Furthermore, two invasive species that might possess serious threat to the endemic plants were observed during the survey: markisa (Passiflora sp.; Passifloraceae) and harendong bulu (Clidemia hirta; Melastomataceae). Based on these results, the present study assigned all the endemic species as Critically Endangered according to the IUCN Red List Category and Criteria. To conserve all the endemic plant species in Mount Salak, several recommendations were given and discussed. 1. Introduction Plants are fundamental part of terrestrial ecosystem and provide support systems for life on earth. For human, plants provide many essential services that underpin human survival and well-being, such as source of food, clothes, timber, medicines, fresh air, clean water, and much more. -

Blair's Rainforest Inventory
Enoggera creek (Herston/Wilston) rainforest inventory Prepared by Blair Bartholomew 28-Jan-02 Botanical Name Common Name: tree, shrub, Derivation (Pronunciation) vine, timber 1. Acacia aulacocarpa Brown salwood, hickory/brush Acacia from Greek ”akakia (A), hê”, the shittah tree, Acacia arabica; (changed to Acacia ironbark/broad-leaved/black/grey which is derived from the Greek “akanth-a [a^k], ês, hê, (akê A)” a thorn disparrima ) wattle, gugarkill or prickle (alluding to the spines on the many African and Asian species first described); aulacocarpa from Greek “aulac” furrow and “karpos” a fruit, referring to the characteristic thickened transverse bands on the a-KAY-she-a pod. Disparrima from Latin “disparrima”, the most unlike, dissimilar, different or unequal referring to the species exhibiting the greatest difference from other renamed species previously described as A aulacocarpa. 2. Acacia melanoxylon Black wood/acacia/sally, light Melanoxylon from Greek “mela_s” black or dark: and “xulon” wood, cut wood, hickory, silver/sally/black- and ready for use, or tree, referring to the dark timber of this species. hearted wattle, mudgerabah, mootchong, Australian blackwood, native ash, bastard myall 3. Acmena hemilampra Broad-leaved lillypilly, blush satin Acmena from Greek “Acmenae” the nymphs of Venus who were very ash, water gum, cassowary gum beautiful, referring to the attractive flowers and fruits. A second source says that Acmena was a nymph dedicated to Venus. This derivation ac-ME-na seems the most likely. Finally another source says that the name is derived from the Latin “Acmena” one of the names of the goddess Venus. Hemilampra from Greek “hemi” half and “lampro”, bright, lustrous or shining, referring to the glossy upper leaf surface. -

Maclurodendron: a New Genus of Rutaceae from Southeast Asia
Maclurodendron: A New Genus of Rutaceae from Southeast Asia T. G. HARTLEY Herbarium Australiense C.S.l.R.O. Division of Plant Industry Canberra, Australia 2601 Abstract The new rutaceous genus Maclurodendron consists of six species and ranges from Sumatra and the Malay Peninsula east to the Philippines and north to Vietnam and Hainan Island. The genus is described and its distinguishing features and apparent relationships are discussed. The six species are keyed, described, and their apparent relationships are outlined. New combinations are made for the names of three species, Maclurodendron porteri, M obovatum, and M oligophlebium, all of which were previously described in the rutaceous genus Acronychia, and three species, M pubescens, M. par· viflorum, and M magnificum, are described as new. Introduction Among the previously described species excluded from Acronychia J. R. & G. Forst. in my revision of that genus (1974) are A. porteri Hook. f., described from W. Malaysia, A. obovata Merr., from the Philippines, and A. oligophlebia Merr., from Hainan Island. These three species plus three others that are undescribed, one from W. Malaysia and two from E. Malaysia, comprise a morphologically isolated group of plants that has not been previously recognised. The purpose of this paper is to give a taxonomic account of this group, which is here described as a new genus. Geographically, these plants range from Sumatra and the Malay Peninsula east to the Philippines and north to Vietnam and Hainan Island (Fig. 1). To Acronychia, which ranges from India to southwestern China and throughout Malesia to eastern Australia and New Caledonia, they are similar in a number of characters, including their opposite leaves, 4-merous flowers, biovulate carpels, and syncarpous, drupaceous fruits. -
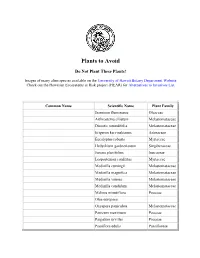
Plants to Avoid
Plants to Avoid Do ot Plant These Plants! Images of many alien species available on the University of Hawaii Botany Department Website Check out the Hawaiian Ecosystems at Risk project (HEAR) for Alternatives to Invasives List Common ame Scientific ame Plant Family Jasminim fluminense Oleaceae Arthrostema ciliatum Melastomataceae Dissotis rotundifolia Melastomataceae Erigeron karvinskianus Asteraceae Eucalyptus robusta Myrtaceae Hedychium gardnerianum Singiberaceae Juncus planifolius Juncaceae Lospostemon confertus Myrtaceae Medinilla cumingii Melastomataceae Medinilla magnifica Melastomataceae Medinilla venosa Melastomataceae Medinilla candidum Melastomataceae Melinis minutiflora Poaceae Olea europaea Oxyspora paniculata Melastomataceae Panicum maximum Poaceae Paspalum urvillei Poaceae Passiflora edulis Passifloreae Phormium tenax Agavaceae Pinus taeda Pinaceae Prosopis pallida Favaceae Pterolepis glomerata Melastomataceae Rhodomyrtus tomentosa Myrtaceae Schefflera actinophylla Araliaceae Syzygium jambos Myrtaceae Australian blackwood Acacia melanoxylon Mimosaceae Australian tree fern Cyathea cooperi Cyatheaceae Australian tree fern Sphaeropteris cooperi Cyatheaceae Beggar's tick, Spanish needle Bidens pilosa Asteraceae California grass Brachiaria mutica Poaceae Chinese banyan, Malayan banyan Ficus mirocarpa Moraceae Chinese violet Asystasia gangetica Acanthaceae Christmasberry, Brazilian pepper Schinus terebinthifolius Anacardiaceae Formosan koa Acacia confusa Mimosaceae German ivy Senecio mikanioides Asteraceae Japanese honeysuckle -

Melastomataceae) of Borneo
BLUMEA 35 (1990) 5-70 Revision of Medinilla(Melastomataceae) of Borneo Jacinto+C. Regalado+Jr. Department of Botany and Plant Pathology, Michigan State University, East Lansing, Michigan 48824,U. S. A.* Summary Forty-eight species ofMedinilla are now known from Borneo, 28 of which are describedas new. At least 20 taxa are known only from one to three collections. Eleven species groups have been of the awaits further of recognized and defined.A more thorough understanding genus study Philip- pine and New Guinea materials. A key to the Bornean species, illustrations of 15 species, and eco- logical notes are provided. Two previously described species are recorded for the first time for Bor- neo: Medinilla succulenta (Blume) Blume, and M. pterocaula Blume. One new combinationand five reductions have been made. Medinilla tawaensis Merrill is transferred to Catanthera; M. caudatifolia Schwartz and M. hasseltii Blume var. subsessilis Schwartz are reduced to M. crassifolia (Reinw. ex Blume) Blume; M. dajakorum Schwartz is reduced to M. corallina Cogn.; M. borneensis Blume and M. motleyi Hook. f. ex Triana are conspecific with M. macrophylla Blume. Introduction Medinilla (Melastomataceae) is a genus of epiphytic and terrestrial shrubs and climbersof the Paleotropics. It includes about 400 species (Shaw, 1973) distributed in Africa, Madagascar, India, Ceylon, Burma, Indochina,southern China, Thailand, the Malay Peninsula, and eastward to the islands of the Malay Archipelago, New Guinea, northern Australia, Micronesia, and Melanesia. It is by far the largest of all melastome genera occurring in Malesia, a floristic region made up of the Malay Peninsula and islands of the Malay Archipelago extending to New Guinea. -

Appelhans Et Al Zanthoxylum
Molecular Phylogenetics and Evolution 126 (2018) 31–44 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Phylogeny and biogeography of the pantropical genus Zanthoxylum and its T closest relatives in the proto-Rutaceae group (Rutaceae) ⁎ Marc S. Appelhansa,b, , Niklas Reichelta, Milton Groppoc, Claudia Paetzolda, Jun Wenb a Department of Systematics, Biodiversity and Evolution of Plants, Albrecht-von-Haller Institute of Plant Sciences, University of Goettingen, Untere Karspuele 2, 37073 Goettingen, Germany b Department of Botany, National Museum of Natural History, Smithsonian Institution, P.O. Box 37012, MRC 166, Washington, DC 20013-7012, USA c Departamento de Biologia, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, São Paulo, Brazil ARTICLE INFO ABSTRACT Keywords: Zanthoxylum L. (prickly ash) is the only genus in the Citrus L. family (Rutaceae) with a pantropical distribution. Bering Land Bridge We present the first detailed phylogenetic and biogeographic study of the genus and its close relatives in the Fagara proto-Rutaceae group. Our phylogenetic analyses based on two plastid and two nuclear markers show that the North Atlantic Land Bridge genus Toddalia Juss. is nested within Zanthoxylum, that earlier generic and intrageneric classifications need Toddalia revision, and that the homochlamydeous flowers of the temperate species of Zanthoxylum are the result of a Transatlantic Disjunction reduction from heterochlamydeous flowers. The biogeographic analyses reveal a Eurasian origin of Zanthoxylum in the Paleocene or Eocene with successive intercontinental or long-range migrations. Zanthoxylum likely crossed the North Atlantic Land Bridges to colonize the Americas in the Eocene, and migrated back to the Old World probably via the Bering Land Bridge in the Oligocene or Miocene. -

Techniques for Breaking Seed Dormancy of Rainforest Species from Genus Acronychia
Liyanage, Offord and Sommerville (2020). Seed Science and Technology, 48, 2, 159-165. https://doi.org/10.15258/sst.2020.48.2.03 Research Note Techniques for breaking seed dormancy of rainforest species from genus Acronychia Ganesha S. Liyanage*, Catherine A. Offord and Karen D. Sommerville The Australian PlantBank, The Royal Botanic Gardens and Domain Trust, Mount Annan, NSW 2567, Australia *Author for correspondence (E-mail: [email protected]) (Submitted February 2020; Accepted March 2020; Published online April 2020) Abstract We tested for dormancy in three species of Acronychia (Rutaceae) occurring in the rainforest in eastern Australia, A. imperforata, A. laevis and A. oblongifolia, by incubating fresh intact seeds on 0.8% water agar for one month at 25/10°C. Four different techniques were then tested for their effect on dormancy: (i) incubation of intact seeds on agar incorporating gibberellic acid (GA3); (ii) seed coat removal (decoating); (iii) scarification near the radicle emergence point (scarification-emergence point); and (iv) scarification opposite the radicle emergence point (scarification-back). Imbibition tests were performed to determine whether dormancy was due to an impermeable seed coat. Germination differed among treatments, but all three species showed a similar pattern. Intact seeds showed < 6% germination after one month indicating the presence of dormancy. Highest germination (> 65%) was observed following scarification-emergence point treatment. Seed coat removal also resulted in increased germination (40-47%), in comparison with intact seeds, but GA3 and scarification-back treatments did not (< 12%). Though the seedcoats of all species were permeable, increased germination responses to decoating and scarification-emergence point treatments suggest scarification is required to clear the radicle emergence point. -

Your Local Native Plant Nursery
Your Local Native Plant Nursery Grow List for Forest Heart Groundcovers Groundcovers cont... Acaena nova-zelandiae Biddy biddy Plumbago zeylandica Native plumbago Artanema fimbriatum Koala bells Pollia crispata Pollia Austrocynoglossum latifolium Forest Hounds tooth Pollia macrophylla Pollia Austromyrtus dulcis Midyim Rostellularia obtusa pink tongue Austromyrtus glabra Midyim Rubus moluccanus Molucca raspberry Brachyscome spp. Daisy Rubus rosifolius Rose leaved raspberry Calotis cuneifolia Burr Daisy Scaevola albida Fan flower Corchorus cunninghamii Native jute Stackhousia spathulata Beach Stackhousia Chrysocephalum apiculatum Yellow buttons Viola banksii Native violet Cullen tenax Emu foot grass Xerochrysum bracteatum Yellow paper daisy Dichondra repens Kidney weed Ferns Enchylaena tomentosa Ruby salt bush Adiantum aethiopicum Common Maidenhair Goodenia arenicola Goodenia Goodenia ovata Goodenia - prostrate form Adiantum formosum Black-stemmed maidenhair Goodenia paniculata Goodenia Adiantum hispidulum Rough maidenhair fern Goodenia rotundifolia Goodenia Asplenium australasicum Birds nest fern Hibbertia aspera Rough guinea flower Blechnum cartilagineum Gristle fern Hibbertia dentata Guinea flower Blechnum indicum Bungwall Hibbertia vestita Rough guinea flower Cyathea cooperi Straw tree fern Isotoma axillaris Australian harebells Doodia aspera Rasp fern Leiocarpa brevicompta Common Sunray Todea barbara King fern Lobelia membranacea Lobelia Lobelia trigonocaulis Forest lobelia Lillies Mazus pumilio Mazus Crinum pedunculata River lily -

Structural Basis for Divergent and Convergent Evolution of Catalytic Machineries in Plant Aromatic Amino Acid Decarboxylase Proteins
Structural basis for divergent and convergent evolution of catalytic machineries in plant aromatic amino acid decarboxylase proteins Michael P. Torrens-Spencea, Ying-Chih Chiangb,1, Tyler Smitha,c, Maria A. Vicenta,d, Yi Wangb, and Jing-Ke Wenga,c,2 aWhitehead Institute for Biomedical Research, Cambridge, MA 02142; bDepartment of Physics, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong; cDepartment of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139; and dDepartment of Biology, Williams College, Williamstown, MA 01267 Edited by Richard A. Dixon, University of North Texas, Denton, TX, and approved March 28, 2020 (received for review November 14, 2019) Radiation of the plant pyridoxal 5′-phosphate (PLP)-dependent aro- such as dopamine and serotonin, from their respective aromatic matic L-amino acid decarboxylase (AAAD) family has yielded an ar- L-amino acid precursors (5). AAAD family enzymes have radi- ray of paralogous enzymes exhibiting divergent substrate preferences ated in plants and insects to yield a number of paralogous en- and catalytic mechanisms. Plant AAADs catalyze either the decar- zymes with variation in both substrate preference and catalytic boxylation or decarboxylation-dependent oxidative deamination mechanism (Fig. 1 A–C and SI Appendix, Fig. S1) (6). In plants, of aromatic L-amino acids to produce aromatic monoamines or L-tryptophan decarboxylases (TDCs) and L-tyrosine decarbox- aromatic acetaldehydes, respectively. These compounds serve as ylases (TyDCs) are canonical AAADs that supply the aromatic key precursors for the biosynthesis of several important classes of monoamine precursors tryptamine and tyramine for the bio- plant natural products, including indole alkaloids, benzylisoquino- line alkaloids, hydroxycinnamic acid amides, phenylacetaldehyde- synthesis of monoterpene indole alkaloids (MIAs) and benzyli- derived floral volatiles, and tyrosol derivatives.