Mammalian Community Recovery from Volcanic Eruptions In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Three-Toed Browsing Horse Anchitherium (Equidae) from the Miocene of Panama
J. Paleonl., 83(3), 2009, pp. 489-492 Copyright © 2009, The Paleontological Society 0022-3360/09/0083-489S03.00 THREE-TOED BROWSING HORSE ANCHITHERIUM (EQUIDAE) FROM THE MIOCENE OF PANAMA BRUCE J. MACFADDEN Florida Museum of Natural History, University of Florida, Gainesville FL 32611, <[email protected]> INTRODUCTION (CRNHT/APL); L, left; M, upper molar; R upper premolar; R, DURING THE Cenozoic, the New World tropics supported a rich right; TRN, greatest transverse width. biodiversity of mammals. However, because of the dense SYSTEMATIC PALEONTOLOGY vegetative ground cover, today relatively little is known about extinct mammals from this region (MacFadden, 2006a). In an Class MAMMALIA Linnaeus, 1758 exception to this generalization, fossil vertebrates have been col- Order PERISSODACTYLA Owen, 1848 lected since the second half of the twentieth century from Neo- Family EQUIDAE Gray, 1821 gene exposures along the Panama Canal. Whitmore and Stewart Genus ANCHITHERIUM Meyer, 1844 (1965) briefly reported on the extinct land mammals collected ANCHITHERIUM CLARENCI Simpson, 1932 from the Miocene Cucaracha Formation that crops out in the Gail- Figures 1, 2, Table 1 lard Cut along the southern reaches of the Canal. MacFadden Referred specimen.—UF 236937, partial palate (maxilla) with (2006b) formally described this assemblage, referred to as the L P1-M3, R P1-P3, and small fragment of anterointernal part of Gaillard Cut Local Fauna (L.E, e.g., Tedford et al., 2004), which P4 (Fig. 1). Collected by Aldo Rincon of the Smithsonian Tropical consists of at least 10 species of carnivores, artiodactyls (also see Research Institute, Republic of Panama, on 15 May 2008. -
![November, 1907.] 56 882 Bulletin American Museum of Natural History](https://docslib.b-cdn.net/cover/9349/november-1907-56-882-bulletin-american-museum-of-natural-history-169349.webp)
November, 1907.] 56 882 Bulletin American Museum of Natural History
56.9,725E( 1182:7) Article XXXV.- REVISION OF THE MIOCENE AND PLIO- CENE EQUII)DE OF NORTH AMERICA. BY JAMES WILLIAMs GIDLEY. With an introductory Note by Henry Fairfield Osborn. INTRODUCTORY NOTE. The American Museum collection of Horses -from the Eocene to the Pleistocene inielusive - now numbers several thousand specimens, includ- ing nearly fifty types and about as many casts of types. It is desired gradually, as opportunity permits, to make this type collection absolutely complete either through originals or casts. The first step towards a thorough understanding of the Equidse is a systematic revision of all the generic and specific names which have been proposed, and of the characters of the valid genera and species, starting with an exact study and comparison of the type specimens. As planned this is being done by co6peration of the writer, of Mr. Walter Granger of the American Museum staff, and especially of Mr. J. W. Gidley, formerly of this Museum, now of the United States National Museum, and the author of the present paper, who has made a specialty of the horse from the Oligo- cene to the' Pleistocene inclusive. The list of these revisions, as completed or in progress, is as follows: Pleistocene. Tooth Characters and Revision of the North American Species of the Genus Equus. By J. W. Gidley. Bull. Am. Mus. Nat. Hist., Vol. XIV, 1901, pp. 91-141, pll. xvii-xxi, and 27 text figures. Miocene and Pliocene. Proper Generic Names of Miocene Horses. By J. W. Gidley. Bull. Amer. Mus. Nat. Hist., Vol. XX, 1904, pp. -

Unit-V Evolution of Horse
UNIT-V EVOLUTION OF HORSE Horses (Equus) are odd-toed hooped mammals belong- ing to the order Perissodactyla. Horse evolution is a straight line evolution and is a suitable example for orthogenesis. It started from Eocene period. The entire evolutionary sequence of horse history is recorded in North America. " Place of Origin The place of origin of horse is North America. From here, horses migrated to Europe and Asia. By the end of Pleis- tocene period, horses became extinct in the motherland (N. America). The horses now living in N. America are the de- scendants of migrants from other continents. Time of Origin The horse evolution started some 58 million years ago, m the beginning of Eocene period of Coenozoic era. The modem horse Equus originated in Pleistocene period about 2 million years ago. Evolutionary Trends The fossils of horses that lived in different periods, show that the body parts exhibited progressive changes towards a particular direction. These directional changes are called evo- lutionary trends. The evolutionary trends of horse evolution are summarized below: 1. Increase in size. 2. Increase in the length of limbs. 3. Increase in the length of the neck. 4. Increase in the length of preorbital region (face). 5. Increase in the length and size of III digit. 6. Increase in the size and complexity of brain. 7. Molarization of premolars. Olfactory bulb Hyracotherium Mesohippus Equus Fig.: Evolution of brain in horse. 8. Development of high crowns in premolars and molars. 9. Change of plantigrade gait to unguligrade gait. 10. Formation of diastema. 11. Disappearance of lateral digits. -

Catalogue Palaeontology Vertebrates (Updated July 2020)
Hermann L. Strack Livres Anciens - Antiquarian Bookdealer - Antiquariaat Histoire Naturelle - Sciences - Médecine - Voyages Sciences - Natural History - Medicine - Travel Wetenschappen - Natuurlijke Historie - Medisch - Reizen Porzh Hervé - 22780 Loguivy Plougras - Bretagne - France Tel.: +33-(0)679439230 - email: [email protected] site: www.strackbooks.nl Dear friends and customers, I am pleased to present my new catalogue. Most of my book stock contains many rare and seldom offered items. I hope you will find something of interest in this catalogue, otherwise I am in the position to search any book you find difficult to obtain. Please send me your want list. I am always interested in buying books, journals or even whole libraries on all fields of science (zoology, botany, geology, medicine, archaeology, physics etc.). Please offer me your duplicates. Terms of sale and delivery: We accept orders by mail, telephone or e-mail. All items are offered subject to prior sale. Please do not forget to mention the unique item number when ordering books. Prices are in Euro. Postage, handling and bank costs are charged extra. Books are sent by surface mail (unless we are instructed otherwise) upon receipt of payment. Confirmed orders are reserved for 30 days. If payment is not received within that period, we are in liberty to sell those items to other customers. Return policy: Books may be returned within 14 days, provided we are notified in advance and that the books are well packed and still in good condition. Catalogue Palaeontology Vertebrates (Updated July 2020) Archaeology AE11189 ROSSI, M.S. DE, 1867. € 80,00 Rapporto sugli studi e sulle scoperte paleoetnologiche nel bacino della campagna romana del Cav. -

Shell Microstructures in Early Cambrian Molluscs
Shell microstructures in Early Cambrian molluscs ARTEM KOUCHINSKY Kouchinsky, A. 2000. Shell microstructures in Early Cambrian molluscs. - Acta Palaeontologica Polonica 45,2, 119-150. The affinities of a considerable part of the earliest skeletal fossils are problematical, but investigation of their microstructures may be useful for understanding biomineralization mechanisms in early metazoans and helpful for their taxonomy. The skeletons of Early Cambrian mollusc-like organisms increased by marginal secretion of new growth lamel- lae or sclerites, the recognized basal elements of which were fibers of apparently aragon- ite. The juvenile part of some composite shells consisted of needle-like sclerites; the adult part was built of hollow leaf-like sclerites. A layer of mineralized prism-like units (low aragonitic prisms or flattened spherulites) surrounded by an organic matrix possibly existed in most of the shells with continuous walls. The distribution of initial points of the prism-like units on a periostracurn-like sheet and their growth rate were mostly regular. The units may be replicated on the surface of internal molds as shallow concave poly- gons, which may contain a more or less well-expressed tubercle in their center. Tubercles are often not enclosed in concave polygons and may co-occur with other types of tex- tures. Convex polygons seem to have resulted from decalcification of prism-like units. They do not co-occur with tubercles. The latter are interpreted as casts of pore channels in the wall possibly playing a role in biomineralization or pits serving as attachment sites of groups of mantle cells. Casts of fibers and/or lamellar units may overlap a polygonal tex- ture or occur without it. -

Barren Ridge FEIS-Volume IV Paleo Tech Rpt Final March
March 2011 BARREN RIDGE RENEWABLE TRANSMISSION PROJECT Paleontological Resources Assessment Report PROJECT NUMBER: 115244 PROJECT CONTACT: MIKE STRAND EMAIL: [email protected] PHONE: 714-507-2710 POWER ENGINEERS, INC. PALEONTOLOGICAL RESOURCES ASSESSMENT REPORT Paleontological Resources Assessment Report PREPARED FOR: LOS ANGELES DEPARTMENT OF WATER AND POWER 111 NORTH HOPE STREET LOS ANGELES, CA 90012 PREPARED BY: POWER ENGINEERS, INC. 731 EAST BALL ROAD, SUITE 100 ANAHEIM, CA 92805 DEPARTMENT OF PALEOSERVICES SAN DIEGO NATURAL HISTORY MUSEUM PO BOX 121390 SAN DIEGO, CA 92112 ANA 032-030 (PER-02) LADWP (MARCH 2011) SB 115244 POWER ENGINEERS, INC. PALEONTOLOGICAL RESOURCES ASSESSMENT REPORT TABLE OF CONTENTS 1.0 INTRODUCTION ........................................................................................................................... 1 1.1 STUDY PERSONNEL ....................................................................................................................... 2 1.2 PROJECT DESCRIPTION .................................................................................................................. 2 1.2.1 Construction of New 230 kV Double-Circuit Transmission Line ........................................ 4 1.2.2 Addition of New 230 kV Circuit ......................................................................................... 14 1.2.3 Reconductoring of Existing Transmission Line .................................................................. 14 1.2.4 Construction of New Switching Station ............................................................................. -

Paleobiology of Archaeohippus (Mammalia; Equidae), a Three-Toed Horse from the Oligocene-Miocene of North America
PALEOBIOLOGY OF ARCHAEOHIPPUS (MAMMALIA; EQUIDAE), A THREE-TOED HORSE FROM THE OLIGOCENE-MIOCENE OF NORTH AMERICA JAY ALFRED O’SULLIVAN A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2002 Copyright 2002 by Jay Alfred O’Sullivan This study is dedicated to my wife, Kym. She provided all of the love, strength, patience, and encouragement I needed to get this started and to see it through to completion. She also provided me with the incentive to make this investment of time and energy in the pursuit of my dream to become a scientist and teacher. That incentive comes with a variety of names - Sylvan, Joanna, Quinn. This effort is dedicated to them also. Additionally, I would like to recognize the people who planted the first seeds of a dream that has come to fruition - my parents, Joseph and Joan. Support (emotional, and financial!) came to my rescue also from my other parents—Dot O’Sullivan, Jim Jaffe and Leslie Sewell, Bill and Lois Grigsby, and Jerry Sewell. To all of these people, this work is dedicated, with love. ACKNOWLEDGMENTS I thank Dr. Bruce J. MacFadden for suggesting that I take a look at an interesting little fossil horse, for always having fresh ideas when mine were dry, and for keeping me moving ever forward. I thank also Drs. S. David Webb and Riehard C. Hulbert Jr. for completing the Triple Threat of Florida Museum vertebrate paleontology. In each his own way, these three men are an inspiration for their professionalism and their scholarly devotion to Florida paleontology. -

Durham Research Online
Durham Research Online Deposited in DRO: 23 May 2017 Version of attached le: Accepted Version Peer-review status of attached le: Peer-reviewed Citation for published item: Betts, Marissa J. and Paterson, John R. and Jago, James B. and Jacquet, Sarah M. and Skovsted, Christian B. and Topper, Timothy P. and Brock, Glenn A. (2017) 'Global correlation of the early Cambrian of South Australia : shelly fauna of the Dailyatia odyssei Zone.', Gondwana research., 46 . pp. 240-279. Further information on publisher's website: https://doi.org/10.1016/j.gr.2017.02.007 Publisher's copyright statement: c 2017 This manuscript version is made available under the CC-BY-NC-ND 4.0 license http://creativecommons.org/licenses/by-nc-nd/4.0/ Additional information: Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in DRO • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full DRO policy for further details. Durham University Library, Stockton Road, Durham DH1 3LY, United Kingdom Tel : +44 (0)191 334 3042 | Fax : +44 (0)191 334 2971 https://dro.dur.ac.uk Accepted Manuscript Global correlation of the early Cambrian of South Australia: Shelly fauna of the Dailyatia odyssei Zone Marissa J. -

Florida Fossil Horse Newsletter
Florida Fossil horse Newsletter Volume 10, Number 1, 1st Half 2001 What's Inside? Fossil Horses On the Road: Archaeohippus and Parahippus Check Out the Bluegrass State Meet Our Artists Volunteers Help the Museum While Enhancing Their Own Education Thunderbeasts, Sexual Selection and Extinction Skeleton "Under Construction" Homeschooling Groups Request More Family Days at Thomas Farm Book Review - The Fossil Vertebrates of Florida Introducing A New Logo For Pony Express's 10th Anniversary Fossil Horses On the Road: Archaeohippus and Parahippus Check Out the Bluegrass State Lexington, Kentucky welcomed Miocene horses at a special event at the Lexington Children's Museum, March 3, 2001. Loaned by the Florida Museum of Natural History, many fossils of the two small prehistoric horses from Thomas Farm are being displayed at the Children's Museum through March and April. Touchable casts of the skulls and feet are also charming the kids, who think the "little horses" are just awesome. At the all-day fossil event, a large display was set up with a case for some of the more fragile horse Seth Woodring, 3, of Winchester, made himself a plaster "fossil" with some help from his mother, fossils. Three Beth, and 5-year-old sister, Rayne. David Stephenson photo (reprinted with permission from tables held fossil Herald Leader) bones and casts, with modern horse bones for comparison. Experts Dr. Teri Lear and Dr. Lenn Harrison, with the Department of Veterinary Science at the University of Kentucky, presented Archaeohippus and Parahippus to the public. Teri has participated in several digs at Thomas Farm, and talked with visitors about the Miocene digs and fossils. -

Middle Miocene Paleoenvironmental Reconstruction of the Central Great Plains from Stable Carbon Isotopes in Large Mammals Willow H
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Dissertations & Theses in Earth and Atmospheric Earth and Atmospheric Sciences, Department of Sciences 7-2017 Middle Miocene Paleoenvironmental Reconstruction of the Central Great Plains from Stable Carbon Isotopes in Large Mammals Willow H. Nguy University of Nebraska-Lincoln, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/geoscidiss Part of the Geology Commons, Paleobiology Commons, and the Paleontology Commons Nguy, Willow H., "Middle Miocene Paleoenvironmental Reconstruction of the Central Great Plains from Stable Carbon Isotopes in Large Mammals" (2017). Dissertations & Theses in Earth and Atmospheric Sciences. 91. http://digitalcommons.unl.edu/geoscidiss/91 This Article is brought to you for free and open access by the Earth and Atmospheric Sciences, Department of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Dissertations & Theses in Earth and Atmospheric Sciences by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. MIDDLE MIOCENE PALEOENVIRONMENTAL RECONSTRUCTION OF THE CENTRAL GREAT PLAINS FROM STABLE CARBON ISOTOPES IN LARGE MAMMALS by Willow H. Nguy A THESIS Presented to the Faculty of The Graduate College at the University of Nebraska In Partial Fulfillment of Requirements For the Degree of Master of Science Major: Earth and Atmospheric Sciences Under the Supervision of Professor Ross Secord Lincoln, Nebraska July, 2017 MIDDLE MIOCENE PALEOENVIRONMENTAL RECONSTRUCTION OF THE CENTRAL GREAT PLAINS FROM STABLE CARBON ISOTOPES IN LARGE MAMMALS Willow H. Nguy, M.S. University of Nebraska, 2017 Advisor: Ross Secord Middle Miocene (18-12 Mya) mammalian faunas of the North American Great Plains contained a much higher diversity of apparent browsers than any modern biome. -
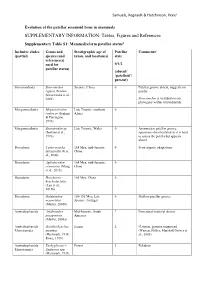
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels, Regnault & Hutchinson, PeerJ Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammaliaform patellar status$ Inclusive clades Genus and Stratigraphic age of Patellar Comments# (partial) species (and taxon, and location(s) state reference(s) used for 0/1/2 patellar status) (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic, China 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska et al., 2004) Sinoconodon is included on our phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji et China al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius (Meng China et al., 2015) Docodonta Docofossor 160 Mya, China 0 brachydactylus (Luo et al., 2015b) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Australosphenida Tachyglossus -

2014BOYDANDWELSH.Pdf
Proceedings of the 10th Conference on Fossil Resources Rapid City, SD May 2014 Dakoterra Vol. 6:124–147 ARTICLE DESCRIPTION OF AN EARLIEST ORELLAN FAUNA FROM BADLANDS NATIONAL PARK, INTERIOR, SOUTH DAKOTA AND IMPLICATIONS FOR THE STRATIGRAPHIC POSITION OF THE BLOOM BASIN LIMESTONE BED CLINT A. BOYD1 AND ED WELSH2 1Department of Geology and Geologic Engineering, South Dakota School of Mines and Technology, Rapid City, South Dakota 57701 U.S.A., [email protected]; 2Division of Resource Management, Badlands National Park, Interior, South Dakota 57750 U.S.A., [email protected] ABSTRACT—Three new vertebrate localities are reported from within the Bloom Basin of the North Unit of Badlands National Park, Interior, South Dakota. These sites were discovered during paleontological surveys and monitoring of the park’s boundary fence construction activities. This report focuses on a new fauna recovered from one of these localities (BADL-LOC-0293) that is designated the Bloom Basin local fauna. This locality is situated approximately three meters below the Bloom Basin limestone bed, a geographically restricted strati- graphic unit only present within the Bloom Basin. Previous researchers have placed the Bloom Basin limestone bed at the contact between the Chadron and Brule formations. Given the unconformity known to occur between these formations in South Dakota, the recovery of a Chadronian (Late Eocene) fauna was expected from this locality. However, detailed collection and examination of fossils from BADL-LOC-0293 reveals an abundance of specimens referable to the characteristic Orellan taxa Hypertragulus calcaratus and Leptomeryx evansi. This fauna also includes new records for the taxa Adjidaumo lophatus and Brachygaulus, a biostratigraphic verifica- tion for the biochronologically ambiguous taxon Megaleptictis, and the possible presence of new leporid and hypertragulid taxa.